synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1 Palladium-catalyzed Coupling Reactions <strong>of</strong> Indoles 45<br />
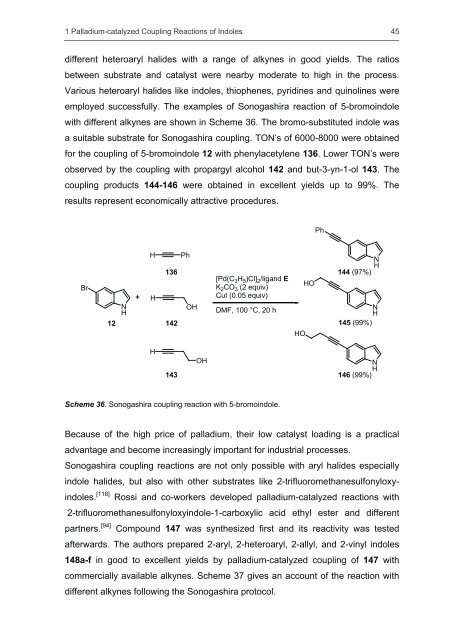
different heteroaryl halides with a range <strong>of</strong> alkynes in good yields. The ratios<br />
between substrate <strong>and</strong> catalyst were nearby moderate to high in the process.<br />
Various heteroaryl halides like <strong>indoles</strong>, thiophenes, pyridines <strong>and</strong> quinolines were<br />
employed successfully. The examples <strong>of</strong> Sonogashira reaction <strong>of</strong> 5-bromoindole<br />
with different alkynes are shown in Scheme 36. The bromo-substituted indole was<br />
a suitable substrate for Sonogashira coupling. TON’s <strong>of</strong> 6000-8000 were obtained<br />
for the coupling <strong>of</strong> 5-bromoindole 12 with phenylacetylene 136. Lower TON’s were<br />
observed by the coupling with propargyl alcohol 142 <strong>and</strong> but-3-yn-1-ol 143. The<br />
coupling products 144-146 were obtained in excellent yields up to 99%. The<br />
results represent economically attr<strong>active</strong> procedures.<br />
Br<br />
12<br />
N<br />
H<br />
H<br />
H<br />
H<br />
136<br />
142<br />
143<br />
Ph<br />
OH<br />
OH<br />
[Pd(C 3H 5)Cl] 2/lig<strong>and</strong> E<br />
K 2CO 3 (2 equiv)<br />
CuI (0.05 equiv)<br />
DMF, 100 °C, 20 h<br />
Scheme 36. Sonogashira coupling reaction with 5-bromoindole.<br />
HO<br />
HO<br />
Ph<br />
144 (97%)<br />
N<br />
H<br />
N<br />
H<br />
145 (99%)<br />
N<br />
H<br />
146 (99%)<br />
Because <strong>of</strong> the high price <strong>of</strong> palladium, their low catalyst loading is a practical<br />
advantage <strong>and</strong> become increasingly important for industrial processes.<br />
Sonogashira coupling reactions are not only possible with aryl halides especially<br />
indole halides, but also with other substrates like 2-trifluoromethanesulfonyloxy<strong>indoles</strong>.<br />
[118] Rossi <strong>and</strong> co-workers developed palladium-catalyzed reactions with<br />
2-trifluoromethanesulfonyloxyindole-1-carboxylic acid ethyl ester <strong>and</strong> different<br />
partners. [94] Compound 147 was synthesized first <strong>and</strong> its reactivity was tested<br />
afterwards. The authors prepared 2-aryl, 2-heteroaryl, 2-allyl, <strong>and</strong> 2-vinyl <strong>indoles</strong><br />
148a-f in good to excellent yields by palladium-catalyzed coupling <strong>of</strong> 147 with<br />
commercially available alkynes. Scheme 37 gives an account <strong>of</strong> the reaction with<br />
different alkynes following the Sonogashira protocol.