synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1 Palladium-catalyzed Coupling Reactions <strong>of</strong> Indoles 48<br />
the 2-alkynylindole derivative 153 in a yield <strong>of</strong> 78%. Under Sonogashira st<strong>and</strong>ard<br />
conditions with Pd(PPh3)4, CuI <strong>and</strong> Et3N they got the key intermediate 153 for the<br />
construction <strong>of</strong> the aspidosperma skeleton.<br />
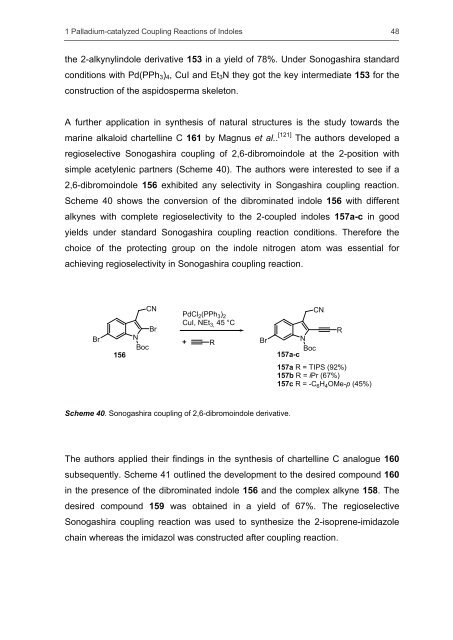
A further application in <strong>synthesis</strong> <strong>of</strong> natural structures is the study towards the<br />
marine alkaloid chartelline C 161 by Magnus et al.. [121] The authors developed a<br />
regioselective Sonogashira coupling <strong>of</strong> 2,6-dibromoindole at the 2-position with<br />
simple acetylenic partners (Scheme 40). The authors were interested to see if a<br />
2,6-dibromoindole 156 exhibited any selectivity in Songashira coupling reaction.<br />
Scheme 40 shows the conversion <strong>of</strong> the dibrominated indole 156 with different<br />
alkynes with complete regioselectivity to the 2-coupled <strong>indoles</strong> 157a-c in good<br />
yields under st<strong>and</strong>ard Sonogashira coupling reaction conditions. Therefore the<br />
choice <strong>of</strong> the protecting group on the indole nitrogen atom was essential for<br />
achieving regioselectivity in Sonogashira coupling reaction.<br />
Br<br />
CN<br />
CN<br />
PdCl2(PPh3) 2<br />
CuI, NEt3, 45 °C<br />
Br<br />
R<br />
N<br />
N<br />
R<br />
Br<br />
Boc<br />
Boc<br />
156 157a-c<br />
157a R = TIPS (92%)<br />
157b R = iPr (67%)<br />
157c R = -C6H4OMe-p (45%)<br />
Scheme 40. Sonogashira coupling <strong>of</strong> 2,6-dibromoindole derivative.<br />
The authors applied their findings in the <strong>synthesis</strong> <strong>of</strong> chartelline C analogue 160<br />
subsequently. Scheme 41 outlined the development to the desired compound 160<br />
in the presence <strong>of</strong> the dibrominated indole 156 <strong>and</strong> the complex alkyne 158. The<br />
desired compound 159 was obtained in a yield <strong>of</strong> 67%. The regioselective<br />
Sonogashira coupling reaction was used to synthesize the 2-isoprene-imidazole<br />
chain whereas the imidazol was constructed after coupling reaction.