synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1 Palladium-catalyzed Coupling Reactions <strong>of</strong> Indoles 62<br />
Cl<br />
CHOH<br />
N<br />
H<br />
Pd(OAc) 2/Xantphos (6 mol%)<br />
Cs 2CO 3 (1.2 equiv)<br />
toluene, 85 °C, 20 h<br />
192 193<br />
1<br />
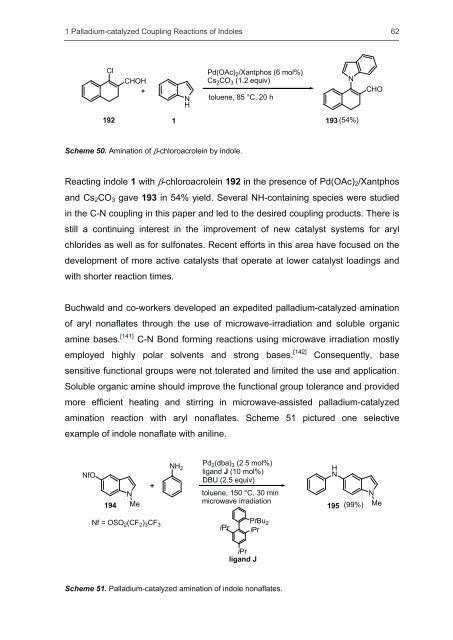
Scheme 50. Amination <strong>of</strong> �-chloroacrolein by indole.<br />
N<br />
(54%)<br />
Reacting indole 1 with �-chloroacrolein 192 in the presence <strong>of</strong> Pd(OAc)2/Xantphos<br />
<strong>and</strong> Cs2CO3 gave 193 in 54% yield. Several NH-containing species were studied<br />
in the C-N coupling in this paper <strong>and</strong> led to the desired coupling products. There is<br />
still a continuing interest in the improvement <strong>of</strong> new catalyst systems for aryl<br />
chlorides as well as for sulfonates. Recent efforts in this area have focused on the<br />
development <strong>of</strong> more <strong>active</strong> catalysts that operate at lower catalyst loadings <strong>and</strong><br />
with shorter reaction times.<br />
Buchwald <strong>and</strong> co-workers developed an expedited palladium-catalyzed amination<br />
<strong>of</strong> aryl nonaflates through the use <strong>of</strong> microwave-irradiation <strong>and</strong> soluble organic<br />
amine bases. [141] C-N Bond forming reactions using microwave irradiation mostly<br />
employed highly polar solvents <strong>and</strong> strong bases. [142] Consequently, base<br />
sensitive functional groups were not tolerated <strong>and</strong> limited the use <strong>and</strong> application.<br />
Soluble organic amine should improve the functional group tolerance <strong>and</strong> provided<br />
more efficient heating <strong>and</strong> stirring in microwave-assisted palladium-catalyzed<br />
amination reaction with aryl nonaflates. Scheme 51 pictured one selective<br />
example <strong>of</strong> indole nonaflate with aniline.<br />
NfO<br />
NH2 Pd2(dba) 3 (2.5 mol%)<br />
lig<strong>and</strong> J (10 mol%)<br />
DBU (2.5 equiv)<br />
H<br />
N<br />
N<br />
toluene, 150 °C, 30 min<br />
194 Me<br />
microwave irradiation<br />
195<br />
Nf = OSO 2(CF 2) 3CF 3<br />
iPr<br />
iPr<br />
lig<strong>and</strong> J<br />
PtBu 2<br />
Scheme 51. Palladium-catalyzed amination <strong>of</strong> indole nonaflates.<br />
iPr<br />
(99%)<br />
CHO<br />
N<br />
Me