synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
synthesis and catalytic functionalization of biologically active indoles
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1 Palladium-catalyzed Coupling Reactions <strong>of</strong> Indoles 41<br />
many researchers have established some flexibility in the conditions, no general<br />
method is yet available for all substrates for this reaction. [112,113]<br />
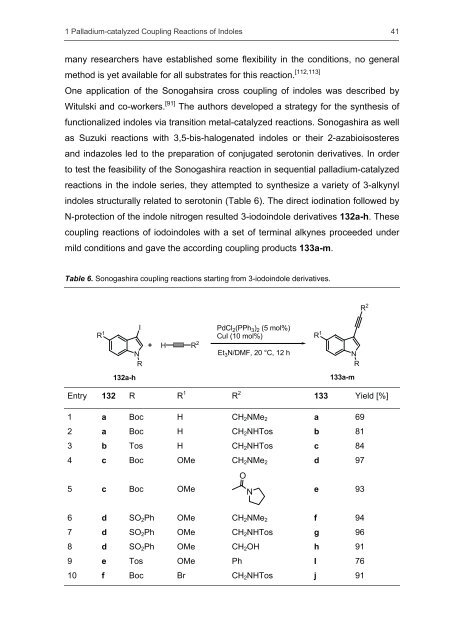
One application <strong>of</strong> the Sonogahsira cross coupling <strong>of</strong> <strong>indoles</strong> was described by<br />
Witulski <strong>and</strong> co-workers. [91] The authors developed a strategy for the <strong>synthesis</strong> <strong>of</strong><br />
functionalized <strong>indoles</strong> via transition metal-catalyzed reactions. Sonogashira as well<br />
as Suzuki reactions with 3,5-bis-halogenated <strong>indoles</strong> or their 2-azabioisosteres<br />
<strong>and</strong> indazoles led to the preparation <strong>of</strong> conjugated serotonin derivatives. In order<br />
to test the feasibility <strong>of</strong> the Sonogashira reaction in sequential palladium-catalyzed<br />
reactions in the indole series, they attempted to synthesize a variety <strong>of</strong> 3-alkynyl<br />
<strong>indoles</strong> structurally related to serotonin (Table 6). The direct iodination followed by<br />
N-protection <strong>of</strong> the indole nitrogen resulted 3-iodoindole derivatives 132a-h. These<br />
coupling reactions <strong>of</strong> iodo<strong>indoles</strong> with a set <strong>of</strong> terminal alkynes proceeded under<br />
mild conditions <strong>and</strong> gave the according coupling products 133a-m.<br />
Table 6. Sonogashira coupling reactions starting from 3-iodoindole derivatives.<br />
R 1<br />
I<br />
N<br />
R<br />
H R 2<br />
PdCl 2(PPh 3) 2 (5 mol%)<br />
CuI (10 mol%)<br />
Et 3N/DMF, 20 °C, 12 h<br />
132a-h 133a-m<br />
Entry 132 R R 1<br />
R 2<br />
R 1<br />
N<br />
R<br />
R 2<br />
133 Yield [%]<br />
1 a Boc H CH2NMe2 a 69<br />
2 a Boc H CH2NHTos b 81<br />
3 b Tos H CH2NHTos c 84<br />
4 c Boc OMe CH2NMe2 d 97<br />
5 c Boc OMe N<br />
O<br />
e 93<br />
6 d SO2Ph OMe CH2NMe2 f 94<br />
7 d SO2Ph OMe CH2NHTos g 96<br />
8 d SO2Ph OMe CH2OH h 91<br />
9 e Tos OMe Ph I 76<br />
10 f Boc Br CH2NHTos j 91