Apoptosis and Degenerative Diseases - Bgbunict.it
Apoptosis and Degenerative Diseases - Bgbunict.it
Apoptosis and Degenerative Diseases - Bgbunict.it
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Apoptosis</strong><strong>and</strong> <strong>Degenerative</strong> diseasesCorso di Biomedicina MolecolareGenomica e Dei Sistemi ComplessiAnno 2009/2010Dott.ssa Laura R<strong>it</strong>a Duro
Cell death modal<strong>it</strong>iesMultiple cell death mechanisms operate in organismsCell death can be classified according to: morphological appearance of the lethal process (that may be apoptotic,necrotic, autophagic or associated w<strong>it</strong>h m<strong>it</strong>osis) enzymological cr<strong>it</strong>eria (w<strong>it</strong>h <strong>and</strong> w<strong>it</strong>hout the involvement of nucleases ordistinct classes of proteases, like caspases or cathepsins) functional aspects (programmed or accidental, physiological orpathological)
Cell death modal<strong>it</strong>ies
<strong>Apoptosis</strong><strong>Apoptosis</strong> is an active cell death thatrequires intact subcellular structures <strong>and</strong>synthesis of phylogenetically conservedproteins. <strong>Apoptosis</strong> arises from the activein<strong>it</strong>iation <strong>and</strong> propagation of a series ofhighly orchestrated specific biochemicalevents leading to the demise of the cell.It can be triggered by numerous types ofcellular damage <strong>and</strong> derangement: genomicinstabil<strong>it</strong>y, cell cycle checkpoint violation,loss of prosurvival signaling.
<strong>Apoptosis</strong><strong>Apoptosis</strong> is characterized by a seriesof dramatic perturbations to thecellular arch<strong>it</strong>ecture that contributenot only to cell death, but also preparecells for removal by phagocytes <strong>and</strong>prevent unwanted immune responses
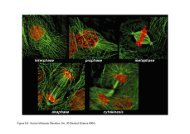
The illustration shows a neuron undergoing acommon form of apoptosis.(A) The healthy neuron has a defined cellmembrane <strong>and</strong> the cytoplasm <strong>and</strong> nucleus, whichcontains DNA, are intact.(B) When apoptosis kicks in, the cell contorts <strong>and</strong>the DNA breaks up.(C) In the final stage of apoptosis, the cell isbroken into membrane-bound pieces. Specializedcells called macrophages or microglia remove thedebris.
The cell begins to shrink following the cleavage of lamins<strong>and</strong> actin filaments in the cytoskeleton (A).The breakdown of chromatin in the nucleus often leads tonuclear condensation <strong>and</strong> in many cases the nuclei ofapoptotic cells take on a "horse-shoe" like appearance (B).Cells continue to shrink (C), packaging themselves into aform that allows for their removal by macrophages.The end stages of apoptosis are often characterised by theappearance of membrane blebs (D) or blisters process.Small vesicles called apoptotic bodies are also sometimesobserved (D, arrow).
<strong>Apoptosis</strong> vs NecrosisDuring necrosis the cellular contents are released uncontrolled into the cell'senvironment which results in damage of surrounding cells <strong>and</strong> a strong inflammatoryresponse in the corresponding tissue.
Removal of dead cellsDying cells that undergo the final stages of apoptosisdisplay phagocytotic molecules, such asphosphatidylserine, on their cell surface.Phosphatidylserine is normally found on the cytosolicsurface of the plasma membrane, but is redistributedduring apoptosis to the extracellular surface. Thesemolecules mark the cell for phagocytosis by cellspossessing the appropriate receptors, such asmacrophages.The removal of dying cells by phagocytes occurs in anorderly manner w<strong>it</strong>hout elic<strong>it</strong>ing an inflammatoryresponse.
Apoptotic PathwaysApoptotic programmed celldeath pathways areactivated by a diverse arrayof cell extrinsic <strong>and</strong> intrinsicsignals, most of which areultimately coupled to theactivation of effectorcaspases
Caspases(cysteine aspartic acidspecificproteases)The caspase family can besubdivided into in<strong>it</strong>iators, whichare able to auto-activate <strong>and</strong>in<strong>it</strong>iate the proteolytic processingof other caspases, <strong>and</strong> effectors,which are activated by othercaspase molecules.The effector caspases cleave thevast major<strong>it</strong>y of substrates duringapoptosis.
Caspases
Caspases coordinate demol<strong>it</strong>ion of key cellular structures <strong>and</strong> organellesEffector caspases (such as caspase-3, -6 <strong>and</strong> -7 inmammals) orchestrate the dismantling of diverse cellstructures through cleavage of specific substrates.Collectively, these proteolytic events produce thephenotypic changes to the cell that are characteristic ofapoptosis.•Cleavage of ICAD releases CAD, which can thencatalyse inter-nucleosomal DNA cleavage.•Proteolysis of proteins at focal adhesion s<strong>it</strong>es <strong>and</strong>cell–cell adhesion s<strong>it</strong>es allows cell detachment <strong>and</strong>retraction.•Caspases also cleave the Golgi-stacking proteinGRASP65 <strong>and</strong> other Golgi proteins, causingfragmentation of the Golgi apparatus.•Important cellular functions such as translation aredisrupted through caspase-mediated proteolysis ofmultiple translation in<strong>it</strong>iation factors (eIFs).•Caspase activ<strong>it</strong>y is required for the exposure ofphosphatidylserine (PS) <strong>and</strong> other phagocytic signalson the cell surface.
Two main pathways of caspase-mediated cell death have beendescribed in mammalsThe “intrinsic”pathway, involvesm<strong>it</strong>ochondria, <strong>and</strong>is triggered <strong>and</strong>controlled bymembers of theBcl- 2proteinfamily.The “extrinsic”pathway ismediated by deathreceptors, asubgroup of thetumor necrosisfactor (TNF)receptorsuperfamily.
Apoptotic PathwaysM<strong>it</strong>ochondrial:activates caspase-9 on a scaffoldformed by Apaf-1 in response tocytochrome c released from damagedm<strong>it</strong>ochondria. This pathway, alsotermed ‘intrinsic’, is primarilyregulated by the Bcl-2 family
Cytochrome c released from damagedm<strong>it</strong>ochondriaInter-nucleosomal DNA cleavage
Apoptotic PathwaysDeath receptor (extrinsic)pathway:apoptosis does not requirecytochrome c release <strong>and</strong> <strong>it</strong>is controlled by deathreceptor signaling
A model of Fas-mediated signaling, caspaseactivation, <strong>and</strong> the induction of a death signalOnce TRADD is recru<strong>it</strong>ed to TNF-R1, <strong>it</strong>functions as an adapter protein to recru<strong>it</strong>several signalling proteins, including TRAF2.TRAF2 seems to be essential for therecru<strong>it</strong>ment of the IKK complex.
Following TRADD binding, three pathways can be in<strong>it</strong>iatedActivation of NF-kBActivation of the MAPK pathwaysInduction of death signaling
The apoptotic response depends on the balance of pro-apoptotic <strong>and</strong>anti-apoptotic signals
The activation of NF-κBThe receptor-interactingprotein (RIP) is recru<strong>it</strong>edvia the death domain tothe TNF–Receptorcomplexupon lig<strong>and</strong>binding <strong>and</strong> can interactw<strong>it</strong>h NF-kB essentialmodulator IKKγ.This results in therecru<strong>it</strong>ment of IKKα <strong>and</strong>IKKβ, which in turnphosphorylate inhib<strong>it</strong>orsof kB (IkB), leading toIkB degradation <strong>and</strong>NF-kB activation
Endoplasmic Reticulum stress can lead to apoptosis
The ER stress response<strong>Apoptosis</strong>, as a last resort, to eliminate infected cells <strong>and</strong> maintain homeostasis
ER stress mediated apoptosis
There is an intensive cross talk between the extrinsic <strong>and</strong> intrinsic pathway, whichenhances an apoptotic signal originally in<strong>it</strong>iated by e<strong>it</strong>her pathway
The BCL-2 familyB-cell lymphoma-2 (BCL-2)-familyproteins have a crucial role in theregulation of apoptosis through theirabil<strong>it</strong>y to regulate m<strong>it</strong>ochondrialcytochrome c release.The Bcl-2 family includesantiapoptotic as well as proapoptoticproteins, <strong>and</strong> can be divided into threemain subfamilies that contain betweenone <strong>and</strong> four ‘BCL-2 Homologyregions’ (BH).
Members of the ‘BH3- only’ subgroup appear to serve asupstream sensors that respond to specific death signals.The BH3-only protein BID is cleaved by caspase-8during extrinsic apoptosis signalling <strong>and</strong> serves to engagea m<strong>it</strong>ochondrial amplification loop in certain cell types.BAD is sw<strong>it</strong>ched on <strong>and</strong> off by <strong>it</strong>s phosphorylation inresponse to growth/survival factors, whilst NOXA <strong>and</strong>PUMA are regulated at the transcriptional level by p53 inresponse to DNA damage.Antiapoptotic Bcl-2 family members serve mainly tosequester BH3-only molecules, thereby preventingBax/Bak activation <strong>and</strong> release of apoptogenic factorsfrom m<strong>it</strong>ochondria.In response to damage or derangement, activators (BIM,BID) activate effectors (BAX, BAK), causingm<strong>it</strong>ochondrial permeabilization <strong>and</strong> comm<strong>it</strong>ment todeath.Antiapoptotic proteins sequester activators to preventtheir contacting effectors, <strong>and</strong> sens<strong>it</strong>izers act as selectiveantagonists of antiapoptotic proteins.Cancer Cell 12, 171–185, August 2007
Implications for human diseaseIn every human being about a hundred thous<strong>and</strong> cells are producedevery second by m<strong>it</strong>osis, <strong>and</strong> a similar number die by apoptosis.It is therefore of crucial importance that the balance between celldeath <strong>and</strong> proliferation is tightly regulated.Consequently, the violation of cellular homeostasis may play aprimary or secondary role in various human diseases, w<strong>it</strong>hessentially too l<strong>it</strong>tle or too much apoptosis leading to proliferativeor degenerative diseases, respectively.
The number of people w<strong>it</strong>h neurodegenerativedisorders is rapidly increasing as the averagelifespan gets longer
NEURODEGENERATIVE DISEASESNeurodegenerativediseases are defined bythe progressive loss ofspecific neuronal cellpopulations
A - In Alzheimer’s disease, neurons in thehippocampus <strong>and</strong> certain regions of the cerebralcortex degenerateB - Huntington’s disease involves the death ofneurons in the striatum, which control bodymovementsIn Parkinson’s disease, dopamine neurons inthe substantia nigra undergo apoptosis.
NEURODEGENERATIVE DISEASESA wide variety of them are characterized by the accumulation ofintracellular or extracellular protein aggregates.Once formed, aggregates tend to be resistant to degradation. Cells haveadapted mechanisms to avoid the accumulation of incorrectly foldedproteins. These mechanisms are molecular chaperones that fold proteinscorrectly <strong>and</strong> the ubiqu<strong>it</strong>in proteasome degradation system that degradesmisfolded or unwanted proteins.In neurodegenerative disorders, these cellular disposal mechanismsbecome ineffective <strong>and</strong>, as a result, misfolded proteins accumulate <strong>and</strong>become toxic for distinctive sets of neurons.This results in a clinical manifestation of disease
Many signals can in<strong>it</strong>iate or ‘trigger’ apoptosis in neurons lack of neurotrophic factor support overactivation of glutamate receptors oxidative stress metabolic stress toxins genetic <strong>and</strong> environmental factors
Roles for altered synaptic signalling in neurodegenerative disordersOveractivation of glutamate receptorsunder cond<strong>it</strong>ions of reduced energyavailabil<strong>it</strong>y or increased oxidative stress(from reactive oxygen species, ROS)results in Ca 2+ influx into postsynapticregions of dendr<strong>it</strong>es.Ca 2+ entering the cytoplasm throughplasma membrane channels <strong>and</strong>endoplasmic reticulum (ER) channelsinduces apoptotic cascades that involvePar-4, pro-apoptotic Bax <strong>and</strong> Bad,<strong>and</strong>/or p53.These factors act on m<strong>it</strong>ochondria toinduce Ca 2+ influx, oxidative stress,opening of permeabil<strong>it</strong>y trans<strong>it</strong>ion pores(PTP) <strong>and</strong> release of cytochrome c.This results in caspase activation <strong>and</strong>execution of the cell death process.
Roles for altered synaptic signalling in neurodegenerative disordersAnti-apoptotic signalling pathwaysare also concentrated in synapticcompartments.For example, activation ofreceptors (R) for neurotrophicfactors (NTF) in axon terminalsstimulates kinase cascades <strong>and</strong>transcription factors <strong>and</strong> increasedproduction of survival-promotingproteins such as Bcl-2, Bcl-xL <strong>and</strong>inhib<strong>it</strong>or of apoptosis proteins(IAPs) (which inhib<strong>it</strong> caspases).
Increasing evidence demonstrates that oxidative stress causesdamage to cell function w<strong>it</strong>h aging <strong>and</strong> is involved in a number ofage-related disorders including atherosclerosis, arthr<strong>it</strong>is, <strong>and</strong>neurodegenerative disorders (amyotrophic lateral sclerosis,Parkinson disease, Huntington disease, <strong>and</strong> Alzheimer disease)
Oxidative stress refers to a state in which free radicals <strong>and</strong> theirproducts are in excess of antioxidant defense mechanismsThis imbalance can occur as a result of increased free radicalproduction or a decrease in antioxidant defenses.
Subsequent events in the neurons are glutamate-induced neurotoxic<strong>it</strong>y <strong>and</strong>increased cytosolic Ca 2+ levels, resulting in activation of Ca 2+ -dependent enzymes.These enzymes produce ROS/RNS, which oxidatively modify nucleic acid, lipid,sugar, <strong>and</strong> protein, leading to nuclear damage, m<strong>it</strong>ochondrial damage, proteasomeinhib<strong>it</strong>ion, <strong>and</strong> ER stress.
Alzheimer’s DiseaseAlzheimer’s disease (AD) is the most common type ofdementia in advanced age.Recent statistics in the Un<strong>it</strong>ed States regarding AD arealarming: 5.3 million people have AD <strong>and</strong> <strong>it</strong> is currently thesixth leading cause of death.<strong>Apoptosis</strong>, 2010
AD is characterized by progressive impairment of cogn<strong>it</strong>ion <strong>and</strong>emotional disturbances that are strongly correlated w<strong>it</strong>h synapticdegeneration <strong>and</strong> death of neurons in Limbic Structures, such as thehippocampus <strong>and</strong> the amygdala, <strong>and</strong> associated regions of thecerebral cortex.
Degenerating neurons show aggregates ofhyperphosphorylated tau protein.A defining feature of Alzheimer’s disease isaccumulation of amyloid plaques formed byaggregates of amyloid-β peptide, a 40-42aminoacid fragment generated byproteolytic processing of the amyloidprecursor protein (APP)Brain tissue section from the hippocampus ofa patient who died w<strong>it</strong>h Alzheimer’s disease
The amyloid precursor protein (APP) is a transmembrane proteinlocated predominantly in the endoplasmic reticulum (ER).APP can be proteolytically processed in two main ways.
Cleavage of APP w<strong>it</strong>hin the Aβ sequence by α-secretase releases a secreted form of APP(sAPPα) from the cell surface.Secreted APPα activates a putative receptor (R)linked to cyclic-GMP production <strong>and</strong> activationof cGMP-dependent protein kinase (PKG). PKGcan then promote opening of K+ channels,resulting in membrane hyperpolarization, <strong>and</strong> canalso activate the transcription factor NF-κB; theseeffects of sAPPα are believed to mediate <strong>it</strong>sneuron survival promoting properties.A second pathway of APP processing involvescleavages of Aβ by β-secretase <strong>and</strong> γ-secretase.This releases Aβ from cells, which, undersu<strong>it</strong>able cond<strong>it</strong>ions (high concentration, oxidizingenvironment), begins to self-aggregate. Aβinduces membrane lipid peroxidation (MLP),which impairs the function of membrane ionmotiveATPases (Na+ <strong>and</strong> Ca2+ pumps) <strong>and</strong>glucose transporters. Neurons are thus vulnerableto apoptosis.
Presenilin-1 (PS-1) is an integral membrane protein located primarily in theendoplasmic reticulum (ER). Presenilins together w<strong>it</strong>h Nicastrin, Aph1 <strong>and</strong> Pen2create a γ-secretase complex that is responsible for the cleavage of APP <strong>and</strong>other integral membrane proteins. Most familial Alzheimer-inducing mutations havebeen identified in the Presenilin genesMutations in PS-1 perturb ER Ca 2+ homeostasis, resulting in increased release of Ca 2+ .The enhanced Ca 2+ release triggers further Ca 2+ influx through Ca 2+ release channels in the plasmamembrane, <strong>and</strong> this altered Ca 2+ homeostasis makes neurons vulnerable to apoptosis <strong>and</strong>exc<strong>it</strong>otoxic<strong>it</strong>y, <strong>and</strong> alters APP processing in a manner that increases Aβ production.
NFTs consist of paired helical filaments (PHF) resulting from thehyper-phosphorylation of the microtubule-binding protein tau.As a microtubuleassociated protein, tauplays an essential rolein maintainingmicrotubule stabil<strong>it</strong>y.In AD neuronal tau isaberrantlyphosphorylated <strong>and</strong>proteolyzed resultingin an impairment of thenormal functions oftau.
The depos<strong>it</strong>ion of Aβ is an early event in thepathogenesis of AD that precedes the formation of NFTs<strong>and</strong> collectively is referred to as the‘‘beta-amyloid hypothesis’’
A series of studies showed that APP can also be cleaved by caspasesin cells <strong>and</strong> in human brain tissueIt was reported that processing of APP atpos<strong>it</strong>ion 720 by caspases increases therate of Aβ secretion.This observation has not beensubstantiated however.In fact accumulating evidence indicatesthat caspase-mediated cleavage of APPdoes not contribute to Aβ production.Cleavage of APP by caspases at pos<strong>it</strong>ion720 removes an internalization motif(Y739ENP) in the intracellular tail of theprotein that has been shown to berequired for Aβ secretion. APP cleavageby caspases would therefore diminishrather than increase Aβ secretion.
If the cleavage of APP by caspases does not increase Aβproduction, does <strong>it</strong> nevertheless participate somehow in theapoptotic process?Processing of APP at pos<strong>it</strong>ion 720 by caspases produces acytoplasmic fragment, called C31, which favors cell death.
The implication of APP cleavage at pos<strong>it</strong>ion 720 has been assessed in vivo.Using in vivo mice model w<strong>it</strong>h the D[720]A caspase-resistant model, <strong>it</strong> wasfound that the mice, desp<strong>it</strong>e similar soluble Aβ accumulation <strong>and</strong> plaqueformation compared to the mice expressing the transgene encoding APP w<strong>it</strong>hthe D720 cleavage s<strong>it</strong>e, did not develop the synaptotoxic<strong>it</strong>y phenotype.Mice prone to Alzheimer-likeneurodegeneration that express acaspase-resistant APP mutant(D720A) are protected fromsynaptotoxic<strong>it</strong>yThis study supports the notion that the formation of senile plaques is not atriggering event for the decreased neuron functional<strong>it</strong>y observed in this disease.
Recent evidence suggests that caspase activation <strong>and</strong> cleavage of tau may linkplaques <strong>and</strong> tangles in AD.
More recently, transgenic mice studies have provideadd<strong>it</strong>ional evidence that caspases play a proximal rolein promoting tangle formation in AD.
To underst<strong>and</strong> the contribution of caspases in disease progression, a tripletransgenic Alzheimer’s mouse model (3xTg-AD) overexpressing the antiapoptoticprotein Bcl-2 was generatedOverexpression of Bcl-2 blocked caspase activation (caspase-9 <strong>and</strong> caspase-3)<strong>and</strong> the cleavage of tau leading to <strong>it</strong>s accumulation w<strong>it</strong>hin neurons.
Desp<strong>it</strong>e the high protein levels oftau, there was l<strong>it</strong>tle evidence forfibrillary tangle formation in3xTg-AD mice overexpressingBcl-2, suggesting that thecaspase-cleavage of tau is acr<strong>it</strong>ical step leading to NFTformationThey also investigated whether a similar effect occurred w<strong>it</strong>h the APP, a substratefor caspase-3-mediated cleavage.Further, desp<strong>it</strong>e the fact the protein levels of APP were significantly higher in 3xTg-AD/Bcl-2-OE mice versus 3xTg-AD mice, there was no evidence for extracellularplaques in these mice.Overexpression of Bcl-2 attenuated the processing of APP <strong>and</strong> tau <strong>and</strong> reduced thenumber of NFTs <strong>and</strong> extracellular depos<strong>it</strong>s of Ab associated w<strong>it</strong>h these animals.These results suggest a significant role for caspase-like proteolytic activ<strong>it</strong>y in theprocessing of APP <strong>and</strong> production of Aβ.
The role of caspases in promoting thepathology associated w<strong>it</strong>h ADStep 1: activation of caspasesStep 2: c-terminal cleavage of APP may facil<strong>it</strong>ate the production of Aβ. This in turn can create avicious feed-forward cycle of Aβ production <strong>and</strong> caspase-3 activation.Step 3: Conversely, caspase-3 cleavage of tau may promote tau aggregation <strong>and</strong> PHF formationthereby linking Aβ to NFTs.Step 4: Aβ depos<strong>it</strong>ion <strong>and</strong> plaque formation.Step 5: Ultimately, the activation of caspases <strong>and</strong> cleavage of cr<strong>it</strong>ical cellular proteins may disruptaxonal <strong>and</strong> dendr<strong>it</strong>ic transport processes leading to cell death <strong>and</strong> neurodegeneration.
Can targeted inhib<strong>it</strong>ion of this class of proteasesprovide an effective means to treat this disease?
Besides approaches to activate or increase the expression of Bcl-2, another potential drugof interest that already has an impressive history is minocycline.It is a tetracycline that has been demonstrated to be effective in the treatment ofHuntington’s disease, Parkinson’s disease as well as in multiple sclerosis.The advantage of minocycline is that <strong>it</strong> is orally available, crosses the blood brain barrier,<strong>and</strong> appears to be safe in humans.Neuroprotection by minocycline appears to be afforded by <strong>it</strong>s abil<strong>it</strong>y to directly inhib<strong>it</strong>the release of cytochrome c <strong>and</strong> prevent the activation of caspase-3.Minocycline has been tested in an animal model of AD <strong>and</strong> was shown to slow neuronalcell death.Minocycline may be a good c<strong>and</strong>idate as a therapeutic agent for AD but add<strong>it</strong>ional studiesare needed.
Parkinson’s DiseaseParkinson's disease (PD) is the second-mostcommon chronic neurological disease ofaging.The cardinal neuropathological features ofPD include progressive loss of dopaminergicterminals in the striatum w<strong>it</strong>h subsequentdeath of neurons in the substantia nigra.Its symptoms are slowness of movements,tremor, rigid<strong>it</strong>y.
Dopamine levels in a normal <strong>and</strong> a Parkinson’s affected neuron
Although subject to intensive research, the etiology of PD isstill enigmatic <strong>and</strong> the treatment is basically symptomatic.Many factors are speculated to operate in the mechanism of celldeath of the nigrostriatal dopaminergic neurons in PD,including oxidative stress <strong>and</strong> cytotoxic<strong>it</strong>y of reactive oxygenspices (ROS), disturbances of intracellular calciumhomeostasis, exogenous <strong>and</strong> endogenous toxins, <strong>and</strong>m<strong>it</strong>ochondrial dysfunction.
Presence of cytoplasmic, α-synuclein pos<strong>it</strong>iveinclusions called Lewy bodies
Defective clearance of modified,toxic proteins via the ubiqu<strong>it</strong>inproteasome system appears toplay a key role in cell death <strong>and</strong>protein aggregation.Aggregates tend to be resistant todegradation.Cells have adapted mechanismsto avoid the accumulation ofincorrectly folded proteins:•Molecular chaperones that fold proteins correctly•Ubiqu<strong>it</strong>in proteasome degradation system that degrades misfolded orunwanted proteins
α-Synuclein is a protein abundantly expressed in presynaptic terminals ofvertebrates. One of <strong>it</strong>s normal functions is to regulate dopamine transporteractiv<strong>it</strong>ies. Different α-synuclein missense mutations (A30P <strong>and</strong> A53T) areassociated w<strong>it</strong>h rare, autosomal dominant, early-onset PD <strong>and</strong> have been shownto form fibrils.Parkin is a 465 amino acid protein which is a component of a multiprotein E3ubiqu<strong>it</strong>in ligase complex which in turn is part of the ubiqu<strong>it</strong>in-proteasome systemthat mediates the targeting of substrate proteins for proteasomal degradation.Mutations in this gene are known to cause a familial form of Parkinson diseaseknown as autosomal recessive juvenile Parkinson disease.
Fas-associated factor 1Caspase activation can be triggered by activation of death receptorssuch as Fas located on the cell surface of target cells.FAF1 is a Fas-binding protein, whose the role in apoptotic cell death isnot well understood. Interestingly the human FAF1 gene has beenlocalized to chromosome 1p32 at the PARK 10 locus, a locus that hasbeen associated w<strong>it</strong>h late-onset Parkinson's disease.Furthermore, in v<strong>it</strong>ro evidence suggests that FAF1 can in<strong>it</strong>iate orenhance Fas-mediated apoptotic cell death
R. Betarbet et al. in this study examined the role of this novelprotein, FAF1, in PD pathogenesis.They demonstrate here that FAF1 expression level isspecifically upregulated in PD <strong>and</strong> in Alzheimer's disease
-FAF1 levels were significantly increased inPD samples as compared to control tissue.-FAF1 upregulation is specific to neuronsw<strong>it</strong>h α-synuclein-pos<strong>it</strong>ive inclusionsStressors, specific to PD, including oxidative stress <strong>and</strong> increased A53Tmutant human α-synuclein expression could significantly increase theexpression levels of endogenous FAF1 indicating that endogenous FAF1levels can be modulated by exogenous insults.These studies clearly demonstrated that FAF1 overexpression induced celldeath. In add<strong>it</strong>ion, FAF1 overexpression could markedly aggravate thetoxic effects of oxidative stress <strong>and</strong> proteasomal inhib<strong>it</strong>ion as suggested byincreased cell death in response to these treatments.FAF1 expression potentiates caspase 3 activation <strong>and</strong> apoptotic cell death.
FAF1 is associated w<strong>it</strong>h PD pathology <strong>and</strong> may have arole in PD pathogenesisFurther studies will be required to better underst<strong>and</strong>mechanisms of FAF1-induced cell death <strong>and</strong>neurodegeneration.
Huntington’s DiseaseHD is an autosomal dominant inher<strong>it</strong>edneurodegenerative disease characterized byprogressive degeneration of GABAergicmedium-sized spiny neurons in the caudatenucleus <strong>and</strong> putamen.Clinically, HD is characterized by the midlifeonset of progressive chorea, cogn<strong>it</strong>ivedecline, <strong>and</strong> psychiatric disturbance.The causative gene in the disease is locatedon chromosome 4p <strong>and</strong> encodes anabnormal CAG triplet repeat expansionresulting in aberrant huntingtin.
CAG trinucleotide expansionsThe specific mechanisms linking the abnormally exp<strong>and</strong>ed huntingtin w<strong>it</strong>hpathology of the disease are not well clarified, however, exc<strong>it</strong>otoxic<strong>it</strong>y,metabolic impairment <strong>and</strong> oxidative stress have been suggested as contributingfactors in the processes leading to neuronal death.
How does mutant huntingtin promote selective degeneration of striatal neurons?Although this is not known, activation of an apoptotic programme is implicated.Huntingtin possesses three caspase s<strong>it</strong>es specific fordifferent caspases. Cleavage by caspases inducesaggregation but this aggregation is more pronouncedw<strong>it</strong>h the mutated form of HuntingtinIn HD, cleavage of mutant htt would release N-terminalfragments w<strong>it</strong>h the potential for increased toxic<strong>it</strong>y <strong>and</strong>accumulation caused by the presence of the exp<strong>and</strong>edpolyglutamine tract. Furthermore accumulation ofexp<strong>and</strong>ed htt fragments in neurons may lead tocorticostriatal dysfunction as an early event in thepathogenesis of HD
The normal form of Huntingtin inhib<strong>it</strong>s caspase-9 but this isdeficient w<strong>it</strong>h poly-glutamine bearing Huntingtin mutants. Bothnormal <strong>and</strong> mutant Huntingtins can prevent activation of caspase-3but the normal forms does <strong>it</strong> more efficiently.Huntingtin can bind HIP1 <strong>and</strong> blocks <strong>it</strong>s pro-apoptotic function.Poly-glutamine bearing Huntingtin mutants bind HIP1 less wellthan the normal form.
Wild-type htt is predominantly cytoplasmic <strong>and</strong> probablyfunctions in vesicle transport, cytoskeletal anchoring, clathrinmediatedendocytosis, neuronal transport or postsynapticsignalling. Htt facil<strong>it</strong>ates dynein-mediated vesicle motil<strong>it</strong>y.Htt may be transported into the nucleus <strong>and</strong> have a role intranscriptional regulation.
The molecular chaperones promote the folding of newly synthesized huntingtin (htt) into a nativestructure. Chaperones can facil<strong>it</strong>ate the recogn<strong>it</strong>ion of abnormal proteins, promoting e<strong>it</strong>her theirrefolding, or ubiqu<strong>it</strong>ination <strong>and</strong> subsequent degradation by the proteasome.The HD mutation induces conformational changes <strong>and</strong> is likely to cause the abnormal folding ofhtt, which, if not corrected by chaperones, leads to the accumulation of misfolded htt in thecytoplasm.Ultimately, toxic<strong>it</strong>y might be elic<strong>it</strong>ed by mutant full-length htt or by cleaved N-terminalfragments, which may form soluble monomers, oligomers or large insoluble aggregates.In the cytoplasm, mutant forms ofhtt may impair the ubiqu<strong>it</strong>in–proteasome system (UPS), leadingto the accumulation of moreproteins that are misfolded. Thesetoxic proteins might also impairnormal vesicle transport <strong>and</strong>clathrin-mediated endocytosis.Also, the presence of mutant httcould activate proapoptoticproteins directly or indirectly bym<strong>it</strong>ochondrial damage, leading togreater cellular toxic<strong>it</strong>y <strong>and</strong> otherdeleterious effects.
NEURODEGENERATIVE DISEASES ANDTHE MAPK INHIBITORSThe M<strong>it</strong>ogen-Activated Protein Kinase (MAPK) family, is involved in thesurvival, proliferation <strong>and</strong> differentiation of nervous cells. Some of theMAPKs promote the differentiation towards the neuron lineage, otherstowards the glial one.The MAPKs are also involved in apoptosis <strong>and</strong> may, therefore, play a role inneurodegeneration.This dual role of MAPKs may make <strong>it</strong> possible to design alternative <strong>and</strong>/orsynergistic approaches to the treatment of degenerative diseases, e<strong>it</strong>her byusing specific inhib<strong>it</strong>ors of the MAPKs involved in apoptosis, or byincreasing the activation of the MAPKs involved in neuronal survival <strong>and</strong>differentiation. The increased activation of pro-differentiative MAPKs canlead to the replacement of damaged neurons by undifferentiated progen<strong>it</strong>ors<strong>and</strong> the slowing down of the disease’s progression.
M<strong>it</strong>ogen-activated protein kinases (MAPKs) are a family of serine-threoninekinases.The MAPK family includes four groups:extracellular signal regulated kinase (ERK),c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK),P38,ERK5MAPKs are activated in response to a wide range of extracellular stimuliincluding growth factors, hormones, cytokines <strong>and</strong> others more related tostress responses (i.e. UV radiation, Xrays, heat <strong>and</strong> osmotic shock), <strong>and</strong> theyare involved in many different cellular processes such as embryogenesis,proliferation, differentiation, transformation <strong>and</strong> apoptosis
MAPK activation occurs through a cascade of three kinaseswhich, after stimulation, are sequentially phosphorylated
MAPKs <strong>and</strong> Alzheimer’s DiseaseThe observation of a colocalization between MAPKs <strong>and</strong> Aβ-amyloid orhyperphosphorylated tau depos<strong>it</strong>s suggests that these proteins may play a rolein AD pathogenesis, <strong>and</strong> many studies have confirmed that MAPKs can bedifferently modulated by Aβ-amyloid administration.Furthermore, transgenic mice overexpressing mutated amyloid precursorproteins show, compared w<strong>it</strong>h wild type controls, increased JNK/SAPK <strong>and</strong>p38 activ<strong>it</strong>y in the cortex at both 7 <strong>and</strong> 12 months of age, in concom<strong>it</strong>ance w<strong>it</strong>hthe increase in amyloid depos<strong>it</strong>ion, tau phosphorylation, <strong>and</strong> loss ofsynaptophysin, an index of synaptic integr<strong>it</strong>y.MAPKs are also involved in tau hyperphosphorylation as observed in bothtransgenic models of AD <strong>and</strong> in brain samples obtained from AD patients. Allthree of the MAPKs participate in tau hyperphosphorylation in AD althoughthey act at different stages of the disease: active ERK1/2 appear at the earlystages while p38 <strong>and</strong> JNK/SAPK act at the later stages. A sequential activationof ERK1/2, JNK/SAPK <strong>and</strong> p38 in susceptible neurons has, therefore, beenrelated to disease progression <strong>and</strong> sever<strong>it</strong>y.
MAPKs <strong>and</strong> Huntington DiseaseMany studies have shown the involvement of MAPKs <strong>and</strong>, in particular, ofJNK/SAPK <strong>and</strong> p38 in different models of HD.The expression of mutated huntingtin w<strong>it</strong>h 48 or 89 polyglutamine repeatsstimulates JNK/SAPK activ<strong>it</strong>y <strong>and</strong> induces apoptotic cell death inHN33cells, an immortalized rat hippocampal cell line, while the expressionof normal huntingtin w<strong>it</strong>h 16 polyglutamine repeats has no toxic effects.In add<strong>it</strong>ion, the JNK/SAPK activation precedes apoptotic cell death whilethe co-expression of the dominant negative mutant form of SEK1 (anupstream JNK/SAPK activator) nearly completely blocks the activation ofJNK/SAPK <strong>and</strong> neuronal apoptosis mediated by mutant huntingtin.On the other h<strong>and</strong>, ERK1/2 are thought to have a protective role, sinceincreased levels of active ERK1/2 protect against cellular death.
MAPKs <strong>and</strong> Parkinson Disease Increasing evidence indicates that MAPKs are involved indopaminergic neuronal degeneration in PD, as has been observed inboth in v<strong>it</strong>ro <strong>and</strong> in vivo models obtained w<strong>it</strong>h exposure to toxicsubstances such as MPTP.In particular, the role of JNK/SAPK <strong>and</strong> p38 pathways seem to beimportant in PD neurodegeneration, while ERK1/2 is more oftenrelated to a neuroprotective action even if <strong>it</strong>s role is not very clear.Moreover parkin is normally involved in ubiqu<strong>it</strong>ination <strong>and</strong>degradation of p38 <strong>and</strong>, therefore, mutations in parkin determine analteration of p38 turnover which suggests that there may be acorrelation between p38 <strong>and</strong> PD pathogenesis.
Current therapies are focused on counteracting thedegenerative events by acting on the molecular mechanismsinvolved in cellular death, or by the exogenous administrationof pro-survival factors.The presence in many areas of both the peripheral <strong>and</strong> centralnervous systems of niches of neural progen<strong>it</strong>ors which c<strong>and</strong>ifferentiate, under specific cond<strong>it</strong>ions, into neurons or glialcells opens up new therapeutic perspectives.







![Glucidi-Lipidi-010210 [modalità compatibilità] - Bgbunict.it](https://img.yumpu.com/41100335/1/190x134/glucidi-lipidi-010210-modalita-compatibilita-bgbunictit.jpg?quality=85)