Guidelines on food fortification with micronutrients - Nutritotal
Guidelines on food fortification with micronutrients - Nutritotal
Guidelines on food fortification with micronutrients - Nutritotal
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong><strong>food</strong> fortificati<strong>on</strong> <strong>with</strong>micr<strong>on</strong>utrientsEdited by Lindsay Allen, Bruno de Benoist,Omar Dary and Richard HurrellI A TFI SPA NFood and Agricultural Organizati<strong>on</strong>of the United Nati<strong>on</strong>s
GUIDELINES ONFOOD FORTIFICATIONWITHMICRONUTRIENTSWorld HealthOrganizati<strong>on</strong>Food and Agricultural Organizati<strong>on</strong>of the United Nati<strong>on</strong>s
<str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong><strong>food</strong> fortificati<strong>on</strong> <strong>with</strong>micr<strong>on</strong>utrientsEdited byLindsay AllenUniversity of California,Davis, CA, United States of AmericaBruno de BenoistWorld Health Organizati<strong>on</strong>,Geneva, SwitzerlandOmar DaryA2Z Outreach – The USAID Micr<strong>on</strong>utrientLeadership and Support and Child Blindness Activity,Washingt<strong>on</strong>, DC, United States of AmericaRichard HurrellSwiss Federal Institute of Technology,Zurich, SwitzerlandWorld HealthOrganizati<strong>on</strong>Food and Agricultural Organizati<strong>on</strong>of the United Nati<strong>on</strong>s
C<strong>on</strong>tentsList of tablesList of figuresForewordPrefaceList of authorsAcknowledgementsAbbreviati<strong>on</strong>sGlossaryxxiiixivxviiixxixxiiixxivxxviPart I. The role of <strong>food</strong> fortificati<strong>on</strong> in the c<strong>on</strong>trol ofmicr<strong>on</strong>utrient malnutriti<strong>on</strong> 1Chapter 1 Micr<strong>on</strong>utrient malnutriti<strong>on</strong>: a public health problem 31.1 Global prevalence of micr<strong>on</strong>utrient malnutriti<strong>on</strong> 31.2 Strategies for the c<strong>on</strong>trol of micr<strong>on</strong>utrient malnutriti<strong>on</strong> 111.2.1 Increasing the diversity of <strong>food</strong>s c<strong>on</strong>sumed 121.2.2 Food fortificati<strong>on</strong> 131.2.3 Supplementati<strong>on</strong> 131.2.4 Public health measures 141.3 Food fortificati<strong>on</strong> in practice 141.3.1 Efficacy trials 151.3.2 Effectiveness evaluati<strong>on</strong>s 171.4 Advantages and limitati<strong>on</strong>s of <strong>food</strong> fortificati<strong>on</strong> as astrategy to combat MNM 20Chapter 2 Food fortificati<strong>on</strong>: basic principles 242.1 Terminology 242.1.1 Food fortificati<strong>on</strong> 242.1.2 Related codex terminology 252.2 Types of fortificati<strong>on</strong> 262.2.1 Mass fortificati<strong>on</strong> 272.2.2 Targeted fortificati<strong>on</strong> 272.2.3 Market-driven fortificati<strong>on</strong> 282.2.4 Other types of fortificati<strong>on</strong> 292.3 Legal c<strong>on</strong>siderati<strong>on</strong>s: mandatory versus voluntaryfortificati<strong>on</strong> 312.3.1 Mandatory fortificati<strong>on</strong> 312.3.2 Voluntary fortificati<strong>on</strong> 332.3.3 Special voluntary fortificati<strong>on</strong> 35iii
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS2.3.4 Criteria governing the selecti<strong>on</strong> of mandatory orvoluntary fortificati<strong>on</strong> 35Part II. Evaluating the public health significance ofmicr<strong>on</strong>utrient malnutriti<strong>on</strong> 39Introducti<strong>on</strong> 41Chapter 3 Ir<strong>on</strong>, vitamin A and iodine 433.1 Ir<strong>on</strong> deficiency and anaemia 433.1.1 Prevalence of deficiency 433.1.2 Risk factors for deficiency 443.1.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 483.2 Vitamin A 483.2.1 Prevalence of deficiency 493.2.2 Risk factors for deficiency 493.2.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 513.3 Iodine 523.3.1 Prevalence of deficiency 523.3.2 Risk factors for deficiency 543.3.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 54Chapter 4 Zinc, folate, vitamin B 12 and other B vitamins, vitamin C,vitamin D, calcium, selenium and fluoride 574.1 Zinc 574.1.1 Prevalence of deficiency 574.1.2 Risk factors for deficiency 594.1.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 614.2 Folate 614.2.1 Prevalence of deficiency 614.2.2 Risk factors for deficiency 634.2.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 634.3 Vitamin B 12 644.3.1 Prevalence of deficiency 654.3.2 Risk factors for deficiency 664.3.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 674.4 Other B vitamins (thiamine, riboflavin, niacin andvitamin B 6 ) 674.4.1 Thiamine 684.4.2 Riboflavin 714.4.3 Niacin 734.4.4 Vitamin B 6 764.5 Vitamin C 784.5.1 Prevalence of deficiency 784.5.2 Risk factors for deficiency 80iv
CONTENTS4.5.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 814.6 Vitamin D 814.6.1 Prevalence of deficiency 824.6.2 Risk factors for deficiency 834.6.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 844.7 Calcium 844.7.1 Prevalence of deficiency 844.7.2 Risk factors for deficiency 854.7.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 864.8 Selenium 864.8.1 Prevalence of deficiency 864.8.2 Risk factors for deficiency 884.8.3 Health c<strong>on</strong>sequences of deficiency andbenefits of interventi<strong>on</strong> 884.9 Fluoride 894.9.1 Prevalence of dental caries 894.9.2 Risk factors for low intakes 904.9.3 Health c<strong>on</strong>sequences of low intakes andbenefits of interventi<strong>on</strong> 904.10 Multiple micr<strong>on</strong>utrient deficiencies 914.10.1 Prevalence and risk factors 914.10.2 Health c<strong>on</strong>sequences and benefits ofinterventi<strong>on</strong> 91Part III. Fortificants: physical characteristics, selecti<strong>on</strong> and use <strong>with</strong>specific <strong>food</strong> vehicles 93Introducti<strong>on</strong> 95Chapter 5 Ir<strong>on</strong>, vitamin A and iodine 975.1 Ir<strong>on</strong> 975.1.1 Choice of ir<strong>on</strong> fortificant 975.1.2 Methods used to increase the amount of ir<strong>on</strong>absorbed from fortificants 1005.1.3 Novel ir<strong>on</strong> fortificants 1025.1.4 Sensory changes 1045.1.5 Experience <strong>with</strong> ir<strong>on</strong> fortificati<strong>on</strong> of specific <strong>food</strong>s 1045.1.6 Safety issues 1105.2 Vitamin A and β-carotene 1115.2.1 Choice of vitamin A fortificant 1115.2.2 Experience <strong>with</strong> vitamin A fortificati<strong>on</strong> ofspecific <strong>food</strong>s 1125.2.3 Safety issues 1175.3 Iodine 1185.3.1 Choice of iodine fortificant 1185.3.2 Experience <strong>with</strong> iodine fortificati<strong>on</strong> of specific<strong>food</strong>s 1195.3.3 Safety issues 122v
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSChapter 6Zinc, folate and other B vitamins, vitamin C, vitamin D, calcium,selenium and fluoride 1246.1 Zinc 1246.1.1 Choice of zinc fortificant 1246.1.2 The bioavailability of zinc 1246.1.3 Methods used to increase zinc absorpti<strong>on</strong> fromfortificants 1256.1.4 Experience <strong>with</strong> zinc fortificati<strong>on</strong> of specific<strong>food</strong>s 1256.2 Folate and other B vitamins 1266.2.1 Choice of vitamin B fortificants 1266.2.2 Experience <strong>with</strong> vitamin B fortificati<strong>on</strong> ofspecific <strong>food</strong>s 1286.2.3 Safety issues 1286.3 Vitamin C (ascorbic acid) 1306.3.1 Choice of vitamin C fortificant 1306.3.2 Experience <strong>with</strong> vitamin C fortificati<strong>on</strong> ofspecific <strong>food</strong>s 1306.4 Vitamin D 1306.4.1 Choice of vitamin D fortificant 1306.4.2 Experience <strong>with</strong> vitamin D fortificati<strong>on</strong> ofspecific <strong>food</strong>s 1306.5 Calcium 1316.5.1 Choice of calcium fortificant 1316.5.2 Experience <strong>with</strong> calcium fortificati<strong>on</strong> 1316.6 Selenium 1336.6.1 Choice of selenium fortificant 1336.6.2 Experience <strong>with</strong> selenium fortificati<strong>on</strong> ofspecific <strong>food</strong>s 1336.7 Fluoride 1346.7.1 Choice of fortificant 1346.7.2 Experience <strong>with</strong> fluoridati<strong>on</strong> 134Part IV. Implementing effective and sustainable <strong>food</strong>fortificati<strong>on</strong> programmes 135Introducti<strong>on</strong> 137Chapter 7 Defining and setting programme 1397.1 Informati<strong>on</strong> needs 1397.1.1 Biochemical and clinical evidence of specificmicr<strong>on</strong>utrient deficiencies 1397.1.2 Dietary patterns 1417.1.3 Usual dietary intakes 1427.2 Defining nutriti<strong>on</strong>al goals: basic c<strong>on</strong>cepts 1427.2.1 The EAR cut-point method 1437.2.2 Dietary reference values: Estimated AverageRequirements, Recommended Nutrient Intakesand upper limits 144vi
CONTENTS7.3 Using the EAR cut-point method to set goals andto evaluate the impact and safety of fortificati<strong>on</strong> 1477.3.1 Deciding <strong>on</strong> an acceptable prevalence oflow intakes 1497.3.2 Calculating the magnitude of micr<strong>on</strong>utrientadditi<strong>on</strong>s 1517.3.3 Adaptati<strong>on</strong>s to the EAR cut-point methodologyfor specific nutrients 1567.3.4 Bioavailability c<strong>on</strong>siderati<strong>on</strong>s 1617.4 Other factors to c<strong>on</strong>sider when deciding fortificati<strong>on</strong>levels 1627.4.1 Safety limits 1637.4.2 Technological limits 1637.4.3 Cost limits 1647.5 Applying the EAR cut-point methodology to mass,targeted and market-driven fortificati<strong>on</strong> interventi<strong>on</strong>s 1647.5.1 Mass fortificati<strong>on</strong> 1667.5.2 Targeted fortificati<strong>on</strong> 1697.5.3 Market-driven fortificati<strong>on</strong> 171Chapter 8 M<strong>on</strong>itoring and evaluati<strong>on</strong> 1788.1 Basic c<strong>on</strong>cepts and definiti<strong>on</strong>s 1788.2 Regulatory m<strong>on</strong>itoring 1808.2.1 Internal m<strong>on</strong>itoring (quality c<strong>on</strong>trol/qualityassurance) 1868.2.2 External m<strong>on</strong>itoring (inspecti<strong>on</strong> and technicalauditing) 1888.2.3 Commercial m<strong>on</strong>itoring 1908.3 Household m<strong>on</strong>itoring 1918.3.1 Aims and objectives 1918.3.2 Methodological c<strong>on</strong>siderati<strong>on</strong>s 1928.4 Impact evaluati<strong>on</strong> 1968.4.1 Impact evaluati<strong>on</strong> design 1968.4.2 Methodological c<strong>on</strong>siderati<strong>on</strong>s 2008.5 What is the minimum every fortificati<strong>on</strong> programmeshould have in terms of a m<strong>on</strong>itoring and evaluati<strong>on</strong>system? 204Chapter 9 Estimating the cost-effectiveness and cost–benefit offortificati<strong>on</strong> 2079.1 Basic c<strong>on</strong>cepts and definiti<strong>on</strong>s 2079.1.1 Cost-effectiveness 2079.1.2 Cost–benefit analysis 2109.2 Informati<strong>on</strong> needs 2109.2.1 Estimating unit costs 2109.2.2 Cost-effectiveness analyses 2139.2.3 Cost–benefit analysis 2159.3 Estimating the cost-effectiveness and cost–benefit ofvitamin A, iodine and ir<strong>on</strong> interventi<strong>on</strong>s:worked examples 216vii
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS9.3.1 Vitamin A supplementati<strong>on</strong>: a cost-effectivenesscalculati<strong>on</strong> 2179.3.2 Iodine: a cost–benefit analysis 2199.3.3 Ir<strong>on</strong> fortificati<strong>on</strong>: a cost–benefit analysis 2209.3.4 Ir<strong>on</strong> supplementati<strong>on</strong>: a cost-effectivenesscalculati<strong>on</strong> 222Chapter 10 Communicati<strong>on</strong>, social marketing, & advocacy in supportof <strong>food</strong> fortificati<strong>on</strong> programmes 22410.1 Communicati<strong>on</strong> strategies: the opti<strong>on</strong>s 22510.1.1 Educati<strong>on</strong> 22610.1.2 Laws, policy and advocacy: communicating<strong>with</strong> policy-makers 22710.1.3 Social marketing 22910.2 Communicati<strong>on</strong> to support social marketing programmes 23010.2.1 Building collaborative partnerships 23210.2.2 Developing messages for government leaders 23410.2.3 Developing messages for industry leaders 23510.2.4 Developing c<strong>on</strong>sumer marketing strategies andc<strong>on</strong>sumer educati<strong>on</strong> 23710.3 Sustaining the programme 238Chapter 11 Nati<strong>on</strong>al <strong>food</strong> law 24011.1 The Internati<strong>on</strong>al c<strong>on</strong>text 24011.2 Nati<strong>on</strong>al <strong>food</strong> law and fortificati<strong>on</strong> 24111.2.1 Forms of <strong>food</strong> law: legislati<strong>on</strong>, regulati<strong>on</strong> andcomplementary measures 24111.2.2 Regulating <strong>food</strong> fortificati<strong>on</strong>: generalc<strong>on</strong>siderati<strong>on</strong>s 24311.3 Mandatory fortificati<strong>on</strong> 24311.3.1 Compositi<strong>on</strong> 24411.3.2 Labelling and advertising 24711.3.3 Trade c<strong>on</strong>siderati<strong>on</strong>s 24911.4 Voluntary fortificati<strong>on</strong> 25011.4.1 Compositi<strong>on</strong> 25111.4.2 Labelling and advertising 25611.4.3 Trade c<strong>on</strong>siderati<strong>on</strong>s 257References 259Further reading 280Annexes 283Annex A Indicators for assessing progress towards the sustainableeliminati<strong>on</strong> of iodine deficiency disorders 285Annex B The internati<strong>on</strong>al resource laboratory for iodine network 287Annex C C<strong>on</strong>versi<strong>on</strong> factors for calculating Estimated AverageRequirements (EARs) from FAO/WHO RecommendedNutrient Intakes (RNIs) 291Annex D A procedure for estimating feasible fortificati<strong>on</strong> levels fora mass fortificati<strong>on</strong> programme 294viii
CONTENTSAnnex E A quality c<strong>on</strong>trol and m<strong>on</strong>itoring system for fortifiedvegetable oils: an example from Morocco 313Annex F The Codex Alimentarius and the World Trade Organizati<strong>on</strong>Agreements 318Index 331ix
List of tablesTable 1.1 Prevalence of the three major micr<strong>on</strong>utrient deficiencies, byWHO regi<strong>on</strong> 4Table 1.2 Micr<strong>on</strong>utrient deficiencies: prevalence, risk factors and healthc<strong>on</strong>sequences 6Table 2.1 Targeted <strong>food</strong> fortificati<strong>on</strong> programmes 28Table 2.2 Foods suited to fortificati<strong>on</strong> at the household level 30Table 3.1 Indicators for assessing ir<strong>on</strong> status at the populati<strong>on</strong> level 45Table 3.2 Criteria for assessing the public health severity of anemia 47Table 3.3 Classificati<strong>on</strong> of usual diets according to their ir<strong>on</strong>bioavailability 47Table 3.4 Indicators for assessing vitamin A status at the populati<strong>on</strong>level 50Table 3.5 Criteria for assessing the public health severity of vitamin Adeficiency 51Table 3.6 Indicators for assessing iodine status at the populati<strong>on</strong> level 53Table 3.7 Criteria for assessing the public health severity of iodinedeficiency 54Table 3.8 The spectrum of iodine deficiency disorders 55Table 4.1 Indicators for assessing zinc status at the populati<strong>on</strong> level 58Table 4.2 Classificati<strong>on</strong> of usual diets according to the potentialbioavailability of their zinc c<strong>on</strong>tent 60Table 4.3 Indicators for assessing folate (vitamin B 9 ) status at thepopulati<strong>on</strong> level 62Table 4.4 Indicators for assessing vitamin B 12 (cobalamin) status at thepopulati<strong>on</strong> level 65Table 4.5 Indicators for assessing thiamine (vitamin B 1 ) status at thepopulati<strong>on</strong> level 69Table 4.6 Proposed criteria for assessing the public health severity ofthiamine deficiency 70Table 4.7 Indicators for assessing riboflavin (vitamin B 2 ) status at thepopulati<strong>on</strong> level 72Table 4.8 Indicators for assessing niacin (nicotinic acid) status at thepopulati<strong>on</strong> level 75Table 4.9 Proposed criteria for assessing public health severity of niacindeficiency 76Table 4.10 Indicators for assessing vitamin B 6 (pyridoxine) status at thepopulati<strong>on</strong> level 77Table 4.11 Indicators for assessing vitamin C status at the populati<strong>on</strong> level 79Table 4.12 Proposed criteria for assessing the public health severity ofvitamin C deficiency 80x
LIST OF TABLESTable 4.13 Indicators for assessing vitamin D status at the populati<strong>on</strong> level 82Table 4.14 Indicators for assessing calcium status at the populati<strong>on</strong> level 85Table 4.15 Indicators for assessing selenium status at the populati<strong>on</strong> level 87Table 4.16 Indicators for assessing fluoride status at the populati<strong>on</strong> level 90Table 5.1 Key characteristics of ir<strong>on</strong> compounds used for <strong>food</strong>fortificati<strong>on</strong> purposes: solubility, bioavailability and cost 98Table 5.2 Suggested ir<strong>on</strong> fortificants for specific <strong>food</strong> vehicles 105Table 5.3 Commercially available forms of vitamin A, their characteristicsand their main applicati<strong>on</strong>s 112Table 5.4 Vitamin A fortificants and their suitability for specific <strong>food</strong>vehicles 113Table 5.5 Examples of vitamin A fortificati<strong>on</strong> programmes 114Table 5.6 Iodine fortificants: chemical compositi<strong>on</strong> and iodine c<strong>on</strong>tent 118Table 5.7 Progress towards universal salt iodizati<strong>on</strong> in WHO regi<strong>on</strong>s,status as of 1999 120Table 6.1 Vitamin B fortificants: physical characteristics and stability 127Table 6.2 Calcium fortificants: physical characteristics 132Table 7.1 FAO/WHO Recommended Nutrient Intakes (RNIs) for selectedpopulati<strong>on</strong> subgroups 145Table 7.2 Estimated Average Requirements (calculated values) based<strong>on</strong> FAO/WHO Recommended Nutrient Intakes 148Table 7.3 Tolerable Upper Intake Levels (ULs) 149Table 7.4 Predicting the effect <strong>on</strong> intake distributi<strong>on</strong>s of adult women offortifying wheat flour <strong>with</strong> different levels of vitamin A 154Table 7.5 Probability of inadequate ir<strong>on</strong> intakes in selected populati<strong>on</strong>subgroups at different ranges of usual intake (mg/day) 158Table 7.6 Prevalence of inadequate ir<strong>on</strong> intakes for menstruating womenc<strong>on</strong>suming a diet from which the average bioavailability ofir<strong>on</strong> is 5%: an example calculati<strong>on</strong> 159Table 7.7 Examples of micr<strong>on</strong>utrients for which the bioavailability of theform used for fortificati<strong>on</strong> differs substantially from theirbioavailability in the usual diet 162Table 7.8 Factors that may limit the amount of fortificants that can beadded to a single <strong>food</strong> vehicle 163Table 7.9 Estimated cost of selected fortificants 165Table 7.10 Examples of levels of micr<strong>on</strong>utrients currently added tostaples and c<strong>on</strong>diments worldwide (mg/kg) 167Table 7.11 Codex Nutrient Reference Values (NRVs) for selectedmicr<strong>on</strong>utrients 172Table 7.12 Energy densities of comm<strong>on</strong> <strong>food</strong> presentati<strong>on</strong>s 174Table 7.13 Calculated maximum micr<strong>on</strong>utrient c<strong>on</strong>tent for a 40 kcal-sizedserving, assuming no other sources of nutrient in the diet 176Table 7.14 Factors for c<strong>on</strong>verting maximum micr<strong>on</strong>utrient amounts for40 kcal-sized servings to maximum amounts for different <strong>food</strong>presentati<strong>on</strong>s and serving sizes 176Table 8.1 Purpose and functi<strong>on</strong> of the various comp<strong>on</strong>ents of m<strong>on</strong>itoringand evaluati<strong>on</strong> systems for fortificati<strong>on</strong> programmes 181Table 8.2 Suggested criteria for measuring success at variousm<strong>on</strong>itoring stages for <strong>food</strong> fortificati<strong>on</strong> programmes 182xi
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTable 8.3 Suggested regulatory m<strong>on</strong>itoring activities for a <strong>food</strong>fortificati<strong>on</strong> programme 183Table 8.4 Suggested household m<strong>on</strong>itoring activities for a <strong>food</strong>fortificati<strong>on</strong> programme 193Table 8.5 Evaluating the impact of fortificati<strong>on</strong> programmes <strong>on</strong> nutriti<strong>on</strong>alstatus: a range of appraoches 198Table 8.6 Impact evaluati<strong>on</strong> of a <strong>food</strong> fortificati<strong>on</strong> programme: suggestedoutcome indicators 201Table 9.1 Hypothetical annual costs of wheat flour fortificati<strong>on</strong> <strong>with</strong> ir<strong>on</strong>and zinc 212Table 9.2 Estimated unit costs of selected micr<strong>on</strong>utrient interventi<strong>on</strong>s 213Table 9.3 Country-specific data required for cost-effectiveness andcost–benefit calculati<strong>on</strong>s, country P 216Table 9.4 Key assumpti<strong>on</strong>s in estimating cost-effectiveness andcost–benefit of selected micr<strong>on</strong>utrient fortificati<strong>on</strong> 217Table 10.1 Nutriti<strong>on</strong> promoti<strong>on</strong> methods defined 225Table 11.1 Relati<strong>on</strong>ship between legal minimum and maximum levels forir<strong>on</strong>, <strong>with</strong> regard to its relative bioavailability from selectedfortificants 247Table A.1 Indicators for m<strong>on</strong>itoring progress towards the sustainableeliminati<strong>on</strong> of iodine deficiency as a public health problem 285Table C.1 C<strong>on</strong>versi<strong>on</strong> factors for calculating Estimated AverageRequirements (EARs) from FAO/WHO RecommendedNutrient Intakes (RNIs) 292Table D.1 C<strong>on</strong>sumpti<strong>on</strong> profile of selected industrially-produced staples 301Table D.2 Recommended compositi<strong>on</strong> of dietary supplements tocomplement fortified <strong>food</strong>s 302Table D.3 Safety limits for vitamin A 303Table D.4 Cost analysis of fortificati<strong>on</strong> <strong>with</strong> vitamin A at the estimatedsafety limits for sugar, oil and wheat flour 304Table D.5 Additi<strong>on</strong>al intake of vitamin A at various levels of c<strong>on</strong>sumpti<strong>on</strong>of fortified <strong>food</strong>s 304Table D.6 Producti<strong>on</strong> parameters for vitamin A fortificati<strong>on</strong> 305Table D.7 Regulatory parameters for vitamin A fortificati<strong>on</strong> 305Table D.8 Safety, technological and cost limits for wheat flour fortificati<strong>on</strong> 307Table D.9 Nutriti<strong>on</strong>al implicati<strong>on</strong>s of wheat flour fortificati<strong>on</strong> 308Table D.10 Producti<strong>on</strong> and regulatory parameters for wheat flourfortificati<strong>on</strong> 309Table D.11 Final formulati<strong>on</strong> for the fortificati<strong>on</strong> of refined wheat flour andestimated associated costs for a hypothetical country 310Table D.12 Estimating the overall cost of the proposed fortificati<strong>on</strong>programme and the annual investment required 311xii
List of figuresFigure 1.1 Effect of ir<strong>on</strong> fortificati<strong>on</strong> of fish sauce <strong>on</strong> the ir<strong>on</strong> status ofn<strong>on</strong>-pregnant anaemic female Vietnamese factory workers 16Figure 1.2 Effect of dual-fortified salt (ir<strong>on</strong> and iodine) <strong>on</strong> ir<strong>on</strong> status ofMoroccan schoolchildren 18Figure 1.3 Effect of flour fortificati<strong>on</strong> <strong>with</strong> folic acid <strong>on</strong> folate status ofCanadian elderly women 19Figure 2.1 The interrelati<strong>on</strong>ships between the levels of coverage andcompliance and the different types of <strong>food</strong> fortificati<strong>on</strong> 27Figure 7.1 An example of a usual intake distributi<strong>on</strong> in which the medianintake is at the RNI or RDA (the formerly-used approach) 144Figure 7.2 An example of a usual intake distributi<strong>on</strong> in which <strong>on</strong>ly 2.5%of the group have intakes below the RNI (RDA) 150Figure 7.3 An example of a usual intake distributi<strong>on</strong> in which 2.5% ofthe group have intakes below the EAR (the recommendedapproach) 150Figure 8.1 A m<strong>on</strong>itoring and evaluati<strong>on</strong> system for fortificati<strong>on</strong>programmes 179Figure 8.2 Suggested frequency and intensity of sampling for m<strong>on</strong>itoringcompliance <strong>with</strong> standards 187Figure 9.1 Cost-effectiveness of micr<strong>on</strong>utrient supplementati<strong>on</strong> andfortificati<strong>on</strong> 209Figure 9.2 Cost-effectiveness of selected interventi<strong>on</strong>s affecting children 209Figure 10.1 Relati<strong>on</strong>ship between individual decisi<strong>on</strong>-making and theperceived costs and benefits of any new behavior, idea orproduct 226xiii
ForewordInterest in micr<strong>on</strong>utrient malnutriti<strong>on</strong> has increased greatly over the last fewyears. One of the main reas<strong>on</strong>s for the increased interest is the realizati<strong>on</strong> thatmicr<strong>on</strong>utrient malnutriti<strong>on</strong> c<strong>on</strong>tributes substantially to the global burden ofdisease. In 2000, the World Health Report 1 identified iodine, ir<strong>on</strong>, vitamin A andzinc deficiencies as being am<strong>on</strong>g the world’s most serious health risk factors. Inadditi<strong>on</strong> to the more obvious clinical manifestati<strong>on</strong>s, micr<strong>on</strong>utrient malnutriti<strong>on</strong>is resp<strong>on</strong>sible for a wide range of n<strong>on</strong>-specific physiological impairments,leading to reduced resistance to infecti<strong>on</strong>s, metabolic disorders, and delayed orimpaired physical and psychomotor development. The public health implicati<strong>on</strong>sof micr<strong>on</strong>utrient malnutriti<strong>on</strong> are potentially huge, and are especially significantwhen it comes to designing strategies for the preventi<strong>on</strong> and c<strong>on</strong>trol ofdiseases such as HIV/AIDS, malaria and tuberculosis, and diet-related chr<strong>on</strong>icdiseases.Another reas<strong>on</strong> for the increased attenti<strong>on</strong> to the problem of micr<strong>on</strong>utrientmalnutriti<strong>on</strong> is that, c<strong>on</strong>trary to previous thinking, it is not uniquely the c<strong>on</strong>cernof poor countries. While micr<strong>on</strong>utrient deficiencies are certainly more frequentand severe am<strong>on</strong>g disadvantaged populati<strong>on</strong>s, they do represent a public healthproblem in some industrialized countries. This is particularly true of iodine deficiencyin Europe, where it was generally assumed to have been eradicated, andof ir<strong>on</strong> deficiency, which is currently the most prevalent micr<strong>on</strong>utrient deficiencyin the world. In additi<strong>on</strong>, the increased c<strong>on</strong>sumpti<strong>on</strong> in industrialized countries(and increasingly in those in social and ec<strong>on</strong>omic transiti<strong>on</strong>) of highly-processedenergy-dense but micr<strong>on</strong>utrient-poor <strong>food</strong>s, is likely to adversely affect micr<strong>on</strong>utrientintake and status.Measures to correct micr<strong>on</strong>utrient deficiencies – at least the major <strong>on</strong>es – are,however, well known, and moreover relatively cheap and easy to implement.The c<strong>on</strong>trol of iodine deficiency disorders through salt iodizati<strong>on</strong>, for example,has been a major accomplishment in public health nutriti<strong>on</strong> over the last30 years.1World health report, 2000. Geneva, World Heath Organizati<strong>on</strong>, 2000.xiv
FOREWORDThe best way of preventing micr<strong>on</strong>utrient malnutriti<strong>on</strong> is to ensure c<strong>on</strong>sumpti<strong>on</strong>of a balanced diet that is adequate in every nutrient. Unfortunately,this is far from being achievable everywhere since it requires universal access toadequate <strong>food</strong> and appropriate dietary habits. From this standpoint, <strong>food</strong> fortificati<strong>on</strong>has the dual advantage of being able to deliver nutrients to large segmentsof the populati<strong>on</strong> <strong>with</strong>out requiring radical changes in <strong>food</strong> c<strong>on</strong>sumpti<strong>on</strong>patterns. In fact, fortificati<strong>on</strong> has been used for more than 80 years in industrializedcountries as a means of restoring micr<strong>on</strong>utrients lost by <strong>food</strong> processing,in particular, some of the B vitamins, and has been a major c<strong>on</strong>tributory factorin the eradicati<strong>on</strong> of diseases associated <strong>with</strong> deficiencies in these vitamins.Because of the increased awareness of the widespread prevalence and harmfuleffects of micr<strong>on</strong>utrient malnutriti<strong>on</strong>, and in c<strong>on</strong>siderati<strong>on</strong> of changes in <strong>food</strong>systems (notably an increased reliance <strong>on</strong> centrally processed <strong>food</strong>s), andsuccessful fortificati<strong>on</strong> experiences in other regi<strong>on</strong>s, increasing numbers ofdeveloping countries are now committed to, or are c<strong>on</strong>sidering, fortificati<strong>on</strong>programmes.With so much accumulated experience, the c<strong>on</strong>diti<strong>on</strong>s under which <strong>food</strong> fortificati<strong>on</strong>can be recommended as a strategic opti<strong>on</strong> for c<strong>on</strong>trolling micr<strong>on</strong>utrientmalnutriti<strong>on</strong> are now better understood. Its limitati<strong>on</strong>s are also well known:<strong>food</strong> fortificati<strong>on</strong> al<strong>on</strong>e cannot correct micr<strong>on</strong>utrient deficiencies when largenumbers of the targeted populati<strong>on</strong>, either because of poverty or locality, havelittle or no access to the fortified <strong>food</strong>, when the level of micr<strong>on</strong>utrient deficiencyis too severe, or when the c<strong>on</strong>current presence of infecti<strong>on</strong>s increases the metabolicdemand for micr<strong>on</strong>utrients. Various safety, technological and cost c<strong>on</strong>siderati<strong>on</strong>scan also place c<strong>on</strong>straints <strong>on</strong> <strong>food</strong> fortificati<strong>on</strong> interventi<strong>on</strong>s. Thus,proper <strong>food</strong> fortificati<strong>on</strong> programme planning not <strong>on</strong>ly requires assessment ofits potential impact <strong>on</strong> the nutriti<strong>on</strong>al status of the populati<strong>on</strong> but also of its feasibilityin a given c<strong>on</strong>text.The success of a fortificati<strong>on</strong> programme can be measured through its publichealth impact and its sustainability. The latter implies an intersectoral approachwhere, in additi<strong>on</strong> to competent nati<strong>on</strong>al public health authorities, research,trade, law, educati<strong>on</strong>, n<strong>on</strong>governmental organizati<strong>on</strong>s and the commercial sectorare all involved in the planning and implementati<strong>on</strong> of the programme. It hastaken time to appreciate the role of the private sector, in particular industry, andthe importance of civil society in this process.These are now fully acknowledgedand this recogniti<strong>on</strong> should strengthen the capability of interventi<strong>on</strong>s to combatmicr<strong>on</strong>utrient malnutriti<strong>on</strong>.The main purpose of these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> is to assist countries in the design andimplementati<strong>on</strong> of appropriate <strong>food</strong> fortificati<strong>on</strong> programmes. Drawing <strong>on</strong>several recent high quality publicati<strong>on</strong>s <strong>on</strong> the subject and <strong>on</strong> programme experience,informati<strong>on</strong> <strong>on</strong> <strong>food</strong> fortificati<strong>on</strong> has been critically analysed and thenxv
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTStranslated into scientifically sound guidelines for applicati<strong>on</strong> in the field. Morespecifically, the <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> provide informati<strong>on</strong> relating to the benefits, limitati<strong>on</strong>s,design, implementati<strong>on</strong>, m<strong>on</strong>itoring, evaluati<strong>on</strong>, cost–benefit and regulati<strong>on</strong>of <strong>food</strong> fortificati<strong>on</strong>, particularly in developing countries. They are intendedto be a resource for governments and agencies that are currently implementing,or c<strong>on</strong>sidering <strong>food</strong> fortificati<strong>on</strong>, and a source of informati<strong>on</strong> for scientists, technologistsand the <strong>food</strong> industry. The <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> are written from a nutriti<strong>on</strong> andpublic health perspective, to provide practical guidance <strong>on</strong> how <strong>food</strong> fortificati<strong>on</strong>should be implemented, m<strong>on</strong>itored and evaluated <strong>with</strong>in the general c<strong>on</strong>textof the need to c<strong>on</strong>trol micr<strong>on</strong>utrient deficiencies in a populati<strong>on</strong>. They are primarilyintended for nutriti<strong>on</strong>-related public health programme managers, butshould also be useful to all those working to c<strong>on</strong>trol micr<strong>on</strong>utrient malnutriti<strong>on</strong>,including industry.The document is organized into four complementary secti<strong>on</strong>s. Part Iintroduces the c<strong>on</strong>cept of <strong>food</strong> fortificati<strong>on</strong> as a potential strategy for the c<strong>on</strong>trolof micr<strong>on</strong>utrient malnutriti<strong>on</strong>. Part II summarizes the prevalence, causesand c<strong>on</strong>sequences of micr<strong>on</strong>utrient deficiencies, and the public health benefitsof micr<strong>on</strong>utrient malnutriti<strong>on</strong> c<strong>on</strong>trol. It lays the groundwork for publichealth pers<strong>on</strong>nel to assess the magnitude of the problem, and the potentialbenefits of fortificati<strong>on</strong>, in their particular situati<strong>on</strong>. Part III provides technicalinformati<strong>on</strong> <strong>on</strong> the various chemical forms of micr<strong>on</strong>utrients that can beused to fortify <strong>food</strong>s, and reviews experience of their use in specific <strong>food</strong>vehicles. Part IV describes the key steps involved in designing, implementingand sustaining fortificati<strong>on</strong> programmes, starting <strong>with</strong> the determinati<strong>on</strong>of the amount of nutrients to be added to <strong>food</strong>s, followed by theimplementati<strong>on</strong> of m<strong>on</strong>itoring and evaluating systems, including qualityc<strong>on</strong>trol/quality assurance procedures, before moving <strong>on</strong> to the estimati<strong>on</strong>of cost-effectiveness and cost–benefit ratios. The importance of, andstrategies for, regulati<strong>on</strong> and internati<strong>on</strong>al harm<strong>on</strong>izati<strong>on</strong>, communicati<strong>on</strong>,advocacy, c<strong>on</strong>sumer marketing and public educati<strong>on</strong> are also explained insome detail.The producti<strong>on</strong> of the <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> has been the result of a l<strong>on</strong>g process thatstarted in 2002. Under the aegis of the World Health Organizati<strong>on</strong> (WHO), anexpert group was established and charged <strong>with</strong> the task of developing a set ofguidelines <strong>on</strong> <strong>food</strong> fortificati<strong>on</strong> practice. A draft versi<strong>on</strong> of the guidelines wasreviewed in 2003 by a multidisciplinary panel of experts who collectively representedthe range of knowledge and experience required for developing suchguidelines. The panel members included experts in public health, nutriti<strong>on</strong> sciencesand <strong>food</strong> technology, from both the public and the private sectors. Afterwards,the draft of the guidelines was circulated am<strong>on</strong>g field nutriti<strong>on</strong>ists andpublic health practiti<strong>on</strong>ers and also tested in a number of countries. All of thexvi
FOREWORDcomments received through this process were c<strong>on</strong>sidered for this finalizedversi<strong>on</strong> of the guidelines.We are all committed to the eliminati<strong>on</strong> of micr<strong>on</strong>utrient malnutriti<strong>on</strong>. Wehope that these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> will help countries to meet this goal and thereforeenable their populati<strong>on</strong> to achieve its full social and ec<strong>on</strong>omic potential.Lindsay AllenBruno de BenoistOmar DaryRichard Hurrellxvii
PrefaceMore than 2 billi<strong>on</strong> people in the world today suffer from micr<strong>on</strong>utrient deficienciescaused largely by a dietary deficiency of vitamins and minerals. Thepublic health importance of these deficiencies lies up<strong>on</strong> their magnitude andtheir health c<strong>on</strong>sequences, especially in pregnant women and young children,as they affect fetal and child growth, cognitive development and resistanceto infecti<strong>on</strong>. Although people in all populati<strong>on</strong> groups in all regi<strong>on</strong>s of theworld may be affected, the most widespread and severe problems are usuallyfound am<strong>on</strong>gst resource poor, <strong>food</strong> insecure and vulnerable households indeveloping countries. Poverty, lack of access to a variety of <strong>food</strong>s, lack ofknowledge of appropriate dietary practices and high incidence of infectiousdiseases are key factors. Micr<strong>on</strong>utrient malnutriti<strong>on</strong> is thus a major impedimentto socio-ec<strong>on</strong>omic development c<strong>on</strong>tributing to a vicious circle of underdevelopmentand to the detriment of already underprivileged groups. It hasl<strong>on</strong>g-ranging effects <strong>on</strong> health, learning ability and productivity and has highsocial and public costs leading to reduced work capacity due to high rates ofillness and disability.Overcoming micr<strong>on</strong>utrient malnutriti<strong>on</strong> is therefore a prec<strong>on</strong>diti<strong>on</strong> for ensuringrapid and appropriate nati<strong>on</strong>al development.This was the c<strong>on</strong>sensus reachedat the FAO/WHO Internati<strong>on</strong>al C<strong>on</strong>ference <strong>on</strong> Nutriti<strong>on</strong> (ICN) in December1992, where 159 countries endorsed the World Declarati<strong>on</strong> <strong>on</strong> Nutriti<strong>on</strong>, pledging“to make all efforts to eliminate ...iodine and vitamin A deficiencies” and“to reduce substantially ...other important micr<strong>on</strong>utrient deficiencies, includingir<strong>on</strong>.” Since then, FAO and WHO have c<strong>on</strong>tinued to work to achieve thisgoal and in doing so have adopted four main strategies improving dietary intakesthrough increased producti<strong>on</strong>, preservati<strong>on</strong> and marketing of micr<strong>on</strong>utrient-rich<strong>food</strong>s combined <strong>with</strong> nutriti<strong>on</strong> educati<strong>on</strong>; <strong>food</strong> fortificati<strong>on</strong>; supplementati<strong>on</strong>;and global public health and other disease c<strong>on</strong>trol measures. Each of these strategieshave a place in eliminating micr<strong>on</strong>utrient malnutriti<strong>on</strong>. For maximumimpact, the right balance or mix of these mutually reinforcing strategies need tobe put in place to ensure access to c<strong>on</strong>sumpti<strong>on</strong> and utilizati<strong>on</strong> of an adequatevariety and quantity of safe, good-quality <strong>food</strong>s for all people of the world.Underpinning these strategies is the realisati<strong>on</strong> that when there is a dietary deficiencyin any <strong>on</strong>e nutrient, there are likely to be other nutrient deficiencies asxviii
PREFACEwell. C<strong>on</strong>sequently in the l<strong>on</strong>g-term, measures for the preventi<strong>on</strong> and c<strong>on</strong>trol ofmicr<strong>on</strong>utrient deficiencies should be based <strong>on</strong> diet diversificati<strong>on</strong> and c<strong>on</strong>sumereducati<strong>on</strong> about how to choose <strong>food</strong>s that provide a balanced diet, including thenecessary vitamins and minerals.These guidelines are meant to assist countries in the design and implementati<strong>on</strong>of appropriate <strong>food</strong> fortificati<strong>on</strong> programmes as part of a comprehensive<strong>food</strong>-based strategy for combating micr<strong>on</strong>utrient deficiencies. Fortificati<strong>on</strong> of<strong>food</strong> can make an important c<strong>on</strong>tributi<strong>on</strong> to the reducti<strong>on</strong> of micr<strong>on</strong>utrientmalnutriti<strong>on</strong> when and where existing <strong>food</strong> supplies and limited access fail toprovide adequate levels of certain nutrients in the diet. To ensure that the targetpopulati<strong>on</strong> will benefit from a <strong>food</strong> fortificati<strong>on</strong> programme, an appropriate <strong>food</strong>vehicle must be selected that is widely c<strong>on</strong>sumed throughout the year by a largeporti<strong>on</strong> of the populati<strong>on</strong> at risk of a particular deficiency. In order to reach differentsegments of the populati<strong>on</strong> who may have different dietary habits, selectingmore than <strong>on</strong>e <strong>food</strong> vehicle may be necessary. Fortificati<strong>on</strong> of a staple <strong>food</strong>affects every<strong>on</strong>e, including the poor, pregnant women, young children and populati<strong>on</strong>sthat can never be completely covered by social services. In additi<strong>on</strong>,fortificati<strong>on</strong> reaches sec<strong>on</strong>dary at-risk groups, such as the elderly and thosewho have an unbalanced diet. Food fortificati<strong>on</strong> is usually socially acceptable,requires no change in <strong>food</strong> habits, does not alter the characteristics of the <strong>food</strong>,can be introduced quickly, can produce nutriti<strong>on</strong>al benefits for the target populati<strong>on</strong>quickly, is safe, and can be a cost-effective way of reaching large targetpopulati<strong>on</strong>s that are at risk of micr<strong>on</strong>utrient deficiency.However, there are limitati<strong>on</strong>s <strong>on</strong> the benefits of fortificati<strong>on</strong> and difficultiesin its implementati<strong>on</strong> and effectiveness. There may, for example, be c<strong>on</strong>cernsraised about the possibility of overdose or a reluctance to fortify <strong>on</strong> human rightsgrounds where c<strong>on</strong>sumer choice may be an issue. There may be reluctance <strong>on</strong>the part of the <strong>food</strong> industry to fortify out of fear of insufficient market demandfor fortified <strong>food</strong>s or c<strong>on</strong>cern about c<strong>on</strong>sumer percepti<strong>on</strong>s that the <strong>food</strong> producthas been altered. Food fortificati<strong>on</strong> also raises producti<strong>on</strong> costs through suchexpenses as initial equipment purchases, equipment maintenance, increased producti<strong>on</strong>staff needs and quality c<strong>on</strong>trol and assurance facilities. Ec<strong>on</strong>omicallymarginalised households may not have access to such <strong>food</strong>s and other vulnerablepopulati<strong>on</strong> groups, particularly children under five years of age, may not beable to c<strong>on</strong>sume large enough quantities of the fortified <strong>food</strong> to satisfy an adequatelevel of their daily requirements. All these issues need to be carefullyassessed and these are discussed in detail.This publicati<strong>on</strong> is a useful guide to assist decisi<strong>on</strong> makers in ensuring thatthe nutriti<strong>on</strong>ally vulnerable and at-risk populati<strong>on</strong>s benefit from <strong>food</strong> fortificati<strong>on</strong>programmes and FAO and WHO would like to express our thanks to allwho have been involved in this process. We reaffirm our support to achieve theMillennium Development Goals set by governments for overall nutriti<strong>on</strong>xix
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSimprovement and will collaborate <strong>with</strong> internati<strong>on</strong>al and nati<strong>on</strong>al agencies so asto accelerate the planning and implementati<strong>on</strong> of comprehensive and sustainable<strong>food</strong> fortificati<strong>on</strong> programmes as <strong>on</strong>e element of nati<strong>on</strong>al nutriti<strong>on</strong> improvementpolicies, plans and programmes.Kraisid T<strong>on</strong>tisirin,Director,Nutriti<strong>on</strong> and C<strong>on</strong>sumer Protecti<strong>on</strong> Divisi<strong>on</strong>,Food and Agriculture Organizati<strong>on</strong>Denise C. Coitinho,Director,Department of Nutriti<strong>on</strong> for Health and Development,World Health Organizati<strong>on</strong>xx
List of authorsLindsay AllenCenter DirectorUSDA, Agricultural Research ServiceWestern Human Nutriti<strong>on</strong> Research CenterUniversity of CaliforniaDavis, California 95616, United States of AmericaBruno de BenoistCoordinator, Micr<strong>on</strong>utrient UnitDepartment of Nutriti<strong>on</strong> for Health and DevelopmentWorld Health Organizati<strong>on</strong>CH 1201, Geneva 27, SwitzerlandOmar DaryFood fortificati<strong>on</strong> specialistA2Z Outreach/The USAID Micr<strong>on</strong>utrient Leadership and Support and ChildBlindness ActivityAcademy for Educati<strong>on</strong>al Development (AED)Washingt<strong>on</strong> D.C. 20009-5721, United States of AmericaRichard HurrellHead, Human Nutriti<strong>on</strong> LaboratoryFood science and Nutriti<strong>on</strong>, Human Nutriti<strong>on</strong>,ETH (Swiss Federal Institute of Technology)CH 8092 Zurich, SwitzerlandSue Hort<strong>on</strong>Professor and Chair Divisi<strong>on</strong> of Social SciencesDepartment of Ec<strong>on</strong>omicsMunk Center for Internati<strong>on</strong>al StudiesUniversity of Tor<strong>on</strong>to (UTSC)Tor<strong>on</strong>to, Ontario M5S 3K7, Canadaxxi
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSJanine LewisPrincipal Nutriti<strong>on</strong>ist, Nutriti<strong>on</strong> and Labelling programmeFood Standards Australia New ZealandPO Box 7186Canberra BC ACT 2610, AustraliaClaudia ParvantaChair and ProfessorDepartment of Social SciencesUniversity of the Sciences in PhiladelphiaPhiladelphia, Pennsylvania, United States of AmericaMohammed RahmaniDépartement des sciences alimentaires et nutriti<strong>on</strong>nellesInstitut agr<strong>on</strong>omique et vétérinaire Hassan IIBP 6202-Instituts10101 Rabat, MoroccoMarie RuelDivisi<strong>on</strong> DirectorFood C<strong>on</strong>sumpti<strong>on</strong> and Nutriti<strong>on</strong> Divisi<strong>on</strong>Internati<strong>on</strong>al Food Policy Research InstituteWashingt<strong>on</strong> D.C. 20006, United States of AmericaBrian Thomps<strong>on</strong>Senior OfficerNutriti<strong>on</strong> and C<strong>on</strong>sumer Protecti<strong>on</strong> Divisi<strong>on</strong>Food and Agriculture Organizati<strong>on</strong>Via delle Terme di Caracalla00100 Rome, Italyxxii
AcknowledgementsSpecial acknowledgement is given to the following experts for their invaluablec<strong>on</strong>tributi<strong>on</strong> to the text and the refinement of the manuscript: Jack Bagriansky,Rune Blomhoff, François Delange, Sean Lynch, Basil Mathioudakis, SuzanneMurphy.These guidelines were also improved by the experts who participated in theTechnical C<strong>on</strong>sultati<strong>on</strong> to review and comment <strong>on</strong> the manuscript c<strong>on</strong>vened byWHO in Geneva in April 2003. Their valuable advice greatly improved theclarity of the text. Those who participated were Maria Anderss<strong>on</strong>, DouglasBalentine, Denise Bienz, André Briend, Rolf Carriere, Ian Darnt<strong>on</strong>-Hill,Jose Chavez, Jose Cordero, Hector Cori, Ines Egli, Dana Faulkner, OlivierF<strong>on</strong>taine, Wilma Freire, Cutberto Garza, Rosalind Gibs<strong>on</strong>, Joyce Greene,Graeme Clugst<strong>on</strong>, Michael Hambidge, Pieter Jooste, Venkatesh Mannar,Reynaldo Martorell, Penelope Nestel, Ibrahim Parvanta, Poul Petersen, PeterRanum, Beatrice Rogers, Richard Smith, Aristide Sagbohan, Bahi Takkouche,Tessa Tan Torres, Robert Tilden, Barbara Underwood,Tina Van Den Briel, AnnaVerster, Emorn Wasantwisut and Trudy Wijnhoven. We acknowledge <strong>with</strong> gratitudeIrwin Rosenberg for chairing the meeting in such a way that the ensuingdebate added much to the c<strong>on</strong>tent of the guidelines.We would like to give a special thanks to Sue Hobbs, Erin McLean, GraceRob and Afrah Shakori who dedicated so much of their time and patience tomake the producti<strong>on</strong> of the guidelines possible and to Victoria Menezes Millerfor her artistic design of the cover illustrati<strong>on</strong>.We would like also to express our deep appreciati<strong>on</strong> to the Government ofLuxembourg for the generous financial support it has provided for the developmentof these guidelines <strong>on</strong> <strong>food</strong> fortificati<strong>on</strong>. This c<strong>on</strong>tributi<strong>on</strong> has enabledthe step-by-step process that was required to establish appropriate normativecriteria for guiding WHO and FAO Member States in the implementati<strong>on</strong> oftheir <strong>food</strong> fortificati<strong>on</strong> programmes. This process included the organizati<strong>on</strong> ofseveral expert meetings to develop the guidelines and a technical c<strong>on</strong>sultati<strong>on</strong>to review and c<strong>on</strong>solidate the guidelines.Lastly, we wish to thank the Global Alliance for Improved Nutriti<strong>on</strong> for itssupport to the publicati<strong>on</strong> of the guidelines.xxiii
Abbreviati<strong>on</strong>sAICDCCHDDALYDFEDRIDRVEAREDTAFAOFFLFNBGAINGDPGMPHACCPICCIDDIDDIIHILOINACGIOMIRLIIVACGIZiNCGLmLLQASmFLMIMMRMNMMTLMWAdequate IntakeCenters for Disease C<strong>on</strong>trolCor<strong>on</strong>ary heart diseaseDisability-adjusted life yearDietary folate equivalentsDietary Recommended IntakeDietary Reference ValueEstimated Average RequirementEthylenediaminetetraacetic acidFood and Agriculture Organizati<strong>on</strong> of the United Nati<strong>on</strong>sFeasible Fortificati<strong>on</strong> LevelFood and Nutriti<strong>on</strong> BoardGlobal Alliance for Improved Nutriti<strong>on</strong>Gross domestic productGood manufacturing practiceHazard analysis critical c<strong>on</strong>trol pointInternati<strong>on</strong>al Council for C<strong>on</strong>trol of Iodine Deficiency DisordersIodine deficiency disordersIodine-induced hyperthroidismInternati<strong>on</strong>al Labour Organizati<strong>on</strong>Internati<strong>on</strong>al Nutriti<strong>on</strong>al Anemia C<strong>on</strong>sultative GroupInstitute of MedicineInternati<strong>on</strong>al Resource Laboratory for IodineInternati<strong>on</strong>al Vitamin A C<strong>on</strong>sultative GroupInternati<strong>on</strong>al Zinc Nutriti<strong>on</strong> C<strong>on</strong>sultative GroupLegal Minimum LevelLot quality assurance samplingMinimum Fortificati<strong>on</strong> LevelMicr<strong>on</strong>utrient InitiativeMaternal mortality rateMicr<strong>on</strong>utrient malnutriti<strong>on</strong>Maximum Tolerable LevelMolecular weightxxiv
ABBREVIATIONSNGONRVPAHOPARPEMQAQCRBVRDARERNIRRSUSTAINTBTUNICEFULUSIVADWFPWHON<strong>on</strong>governmental organizati<strong>on</strong>Nutrient Reference ValuePan American Health Organizati<strong>on</strong>Populati<strong>on</strong> attributable riskProtein–energy malnutriti<strong>on</strong>Quality assuranceQuality c<strong>on</strong>trolRelative bioavailabilityRecommended Dietary AllowanceRetinol equivalentsRecommended Nutrient IntakeRelative riskSharing United States Technology to Aid in the Improvement ofNutriti<strong>on</strong>(Agreement <strong>on</strong>) Technical Barriers to TradeUnited Nati<strong>on</strong>s Children’s FundTolerable Upper Intake LevelUniversal salt iodizati<strong>on</strong>Vitamin A deficiencyWorld Food ProgrammeWorld Health Organizati<strong>on</strong>xxv
GlossaryThe Average Intake (AI) is a recommended intake value based <strong>on</strong> observedor experimentally determined approximati<strong>on</strong>s or estimates of nutrient intakeby a group or groups of apparently healthy people that are assumed to beadequate.Cost limit refers to the maximum acceptable increment in price of a <strong>food</strong> dueto fortificati<strong>on</strong>.A Dietary Recommended Intake (DRI) is a quantitative estimate of a nutrientintake that is used as a reference value for planning and assessing dietsfor apparently healthy people. Examples include AIs, EARs, RDAs and ULs.Effectiveness refers to the impact of an interventi<strong>on</strong> in practice. Compared toefficacy, the effectiveness of a fortificati<strong>on</strong> programme will be limited byfactors such as n<strong>on</strong>- or low c<strong>on</strong>sumpti<strong>on</strong> of the fortified <strong>food</strong>.Efficacy refers to the capacity of an interventi<strong>on</strong> such as fortificati<strong>on</strong> to achievethe desired impact under ideal circumstances. This usually refers to experimental,well-supervised interventi<strong>on</strong> trials.Enrichment is syn<strong>on</strong>ymous <strong>with</strong> fortificati<strong>on</strong> and refers to the additi<strong>on</strong> ofmicr<strong>on</strong>utrients to a <strong>food</strong> irrespective of whether the nutrients were originallyin the <strong>food</strong> before processing or not.Essential micr<strong>on</strong>utrient refers to any micr<strong>on</strong>utrient, which is needed forgrowth and development and the maintenance of healthy life, that is normallyc<strong>on</strong>sumed as a c<strong>on</strong>stituent of <strong>food</strong> and cannot be synthesized in adequateamounts by the body.The Estimated Average Requirement (EAR) is the average (median) dailynutrient intake level estimated to meet the needs of half the healthy individualsin a particular age and gender group. The EAR is used to derive theRecommended Dietary Allowance.Evaluati<strong>on</strong> refers to the assessment of the effectiveness and impact of the programme<strong>on</strong> the targeted populati<strong>on</strong>. The aim of an evaluati<strong>on</strong> is to provideevidence that the programme is achieving its nutriti<strong>on</strong>al goals.Feasible Fortificati<strong>on</strong> Level (FFL) is that which is determined, subject to costxxvi
GLOSSARYand technological c<strong>on</strong>straints, as the level that will provide the greatestnumber of at-risk individual <strong>with</strong> an adequate intake <strong>with</strong>out causing an unacceptablerisk of excess intakes in the whole populati<strong>on</strong>.Food commodities are staple <strong>food</strong>s, c<strong>on</strong>diments and milk.Fortificati<strong>on</strong> is the practice of deliberately increasing the c<strong>on</strong>tent of an essentialmicr<strong>on</strong>utrient, i.e. vitamins and minerals (including trace elements) in a<strong>food</strong>, so as to improve the nutriti<strong>on</strong>al quality of the <strong>food</strong> supply and providea public health benefit <strong>with</strong> minimal risk to health.Legal Minimum level (LmL) is the minimum amount of micr<strong>on</strong>utrient that afortified <strong>food</strong> must c<strong>on</strong>tain according to nati<strong>on</strong>al regulati<strong>on</strong>s and standards.This value is estimated by adding the intrinsic c<strong>on</strong>tent of a micr<strong>on</strong>utrient inthe <strong>food</strong> to the selected level of fortificati<strong>on</strong>.Market-driven fortificati<strong>on</strong> refers to the situati<strong>on</strong> where the <strong>food</strong> manufacturertakes the initiative to add <strong>on</strong>e or more micr<strong>on</strong>utrients to processed <strong>food</strong>s,usually <strong>with</strong>in regulatory limits, in order to increase sales and profitability.Mass fortificati<strong>on</strong> refers to the additi<strong>on</strong> of micr<strong>on</strong>utrients to <strong>food</strong>s comm<strong>on</strong>lyc<strong>on</strong>sumed by the general public, such as cereals, c<strong>on</strong>diments and milk.Maximum Tolerable Level (MTL) is the maximum micr<strong>on</strong>utrient c<strong>on</strong>tent thata fortified <strong>food</strong> can present as it is established in <strong>food</strong> law, in order to minimizethe risk of excess intake. It should coincide or be lower than the safetylimit.Minimum Fortificati<strong>on</strong> Level (mFL) is the level calculated by reducing theFeasible Fortificati<strong>on</strong> Level by three standards deviati<strong>on</strong>s (or coefficients ofvariati<strong>on</strong>) of the fortificati<strong>on</strong> process, in order that the average coincides oris lower than the calculated Feasible Fortificati<strong>on</strong> Level.M<strong>on</strong>itoring refers to the c<strong>on</strong>tinuous collecti<strong>on</strong> and review of informati<strong>on</strong> <strong>on</strong>programme implementati<strong>on</strong> activities for the purposes of identifying problems(such as n<strong>on</strong>-compliance) and taking corrective acti<strong>on</strong>s so that the programmefulfils its stated objectives.Nutriti<strong>on</strong>al equivalence is achieved when an essential nutrient is added to aproduct that is designed to resemble a comm<strong>on</strong> <strong>food</strong> in appearance, texture,flavour and odour in amounts such that the substitute product has a similarnutritive value, in terms of the amount and bioavailability of the added essentialnutrient.Nutrient Reference Values (NRVs) are dietary reference values defined by theCodex Alimentarius Commissi<strong>on</strong> <strong>with</strong> the aim of harm<strong>on</strong>izing the labellingof processed <strong>food</strong>s. It is a value applicable to all members of the family agedxxvii
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS3 years and over. These values are c<strong>on</strong>stantly reviewed based <strong>on</strong> advances inscientific knowledge.Nutrient requirement refers to the lowest c<strong>on</strong>tinuing intake level of a nutrientthat will maintain a defined level of nutriture in an individual for a given criteri<strong>on</strong>of nutriti<strong>on</strong>al adequacy.Processed <strong>food</strong>s are those in which <strong>food</strong> raw materials have been treated industriallyso as to preserve them. Some may be formulated by mixing several differentingredients.A premix is a mixture of a micr<strong>on</strong>utrient(s) and another ingredient, oftenthe same <strong>food</strong> that is to be fortified, that is added to the <strong>food</strong> vehicle toimprove the distributi<strong>on</strong> of the micr<strong>on</strong>utrient mix <strong>with</strong>in the <strong>food</strong> matrix andto reduce the separati<strong>on</strong> (segregati<strong>on</strong>) between the <strong>food</strong> and micr<strong>on</strong>utrientparticles.Quality assurance (QA) refers to the implementati<strong>on</strong> of planned and systematicactivities necessary to ensure that products or services meet quality standards.The performance of quality assurance can be expressed numericallyas the results of quality c<strong>on</strong>trol exercises.Quality c<strong>on</strong>trol (QC) refers to the techniques and assessments used to documentcompliance of the product <strong>with</strong> established technical standards, throughthe use of objective and measurable indicators.Relative bioavailability is used to rank the absorbability of a nutrient by comparingits absorbability <strong>with</strong> that of a reference nutrient that is c<strong>on</strong>sidered ashaving the most efficient absorbability.Restorati<strong>on</strong> is the additi<strong>on</strong> of essential nutrients to <strong>food</strong>s to restore amountsoriginally present in the natural product, but unavoidably lost during processing(such as milling), storage or handling.Recommended Dietary Allowances (RDAs) are defined by the United StatesFood and Nutriti<strong>on</strong> Board and are c<strong>on</strong>ceptually the same as the RecommendedNutrient Intake (RNI), but may have a slightly different values forsome micr<strong>on</strong>utrients.The Recommended Nutrient Intake (RNI) is the daily intake that meets thenutrient requirements of almost all apparently healthy individuals in an ageandsex-specific populati<strong>on</strong> group. It is set at the Estimated Average Requirementplus 2 standard deviati<strong>on</strong>s.Safety limit is the greatest amount of a micr<strong>on</strong>utrient that can be safely addedto specific <strong>food</strong>s. It c<strong>on</strong>siders the UL for the nutrient and the 95 th percentileof c<strong>on</strong>sumpti<strong>on</strong> of a <strong>food</strong>, and makes allowances for the fact that thexxviii
GLOSSARYnutrient is also c<strong>on</strong>sumed in unfortified <strong>food</strong>s, and may be lost during storageand distributi<strong>on</strong>, and/or cooking.Targeted fortificati<strong>on</strong> refers to the fortificati<strong>on</strong> of <strong>food</strong>s designed for specificpopulati<strong>on</strong> subgroups, such as complementary weaning <strong>food</strong>s for infants.The technological limit is the maximum level of micr<strong>on</strong>utrient additi<strong>on</strong> thatdoes not change the organoleptic or physical properties of the <strong>food</strong>.The Tolerable Upper Intake Level (UL) is to the highest average daily nutrientintake level unlikely to pose risk of adverse health effects to almost all(97.5%) apparently healthy individuals in an age- and sex-specific populati<strong>on</strong>group.Universal fortificati<strong>on</strong> is equivalent to mass fortificati<strong>on</strong>.Universal salt iodizati<strong>on</strong> (USI) refers to the additi<strong>on</strong> of iodine to all salt forboth human and animal c<strong>on</strong>sumpti<strong>on</strong>.Usual intake refers to an individual’s average intake over a relatively l<strong>on</strong>g periodof time.xxix
PA RT IThe role of <strong>food</strong> fortificati<strong>on</strong>in the c<strong>on</strong>trol ofmicr<strong>on</strong>utrient malnutriti<strong>on</strong>
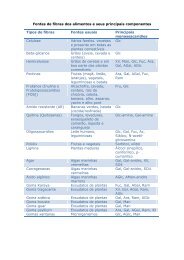
CHAPTER 1Micr<strong>on</strong>utrient malnutriti<strong>on</strong>: a publichealth problem1.1 Global prevalence of micr<strong>on</strong>utrient malnutriti<strong>on</strong>Micr<strong>on</strong>utrient malnutriti<strong>on</strong> (MNM) is widespread in the industrialized nati<strong>on</strong>s,but even more so in the developing regi<strong>on</strong>s of the world. It can affect all agegroups, but young children and women of reproductive age tend to be am<strong>on</strong>gthose most at risk of developing micr<strong>on</strong>utrient deficiencies. Micr<strong>on</strong>utrient malnutriti<strong>on</strong>has many adverse effects <strong>on</strong> human health, not all of which are clinicallyevident. Even moderate levels of deficiency (which can be detected bybiochemical or clinical measurements) can have serious detrimental effects <strong>on</strong>human functi<strong>on</strong>. Thus, in additi<strong>on</strong> to the obvious and direct health effects, theexistence of MNM has profound implicati<strong>on</strong>s for ec<strong>on</strong>omic development andproductivity, particularly in terms of the potentially huge public health costs andthe loss of human capital formati<strong>on</strong>.Worldwide, the three most comm<strong>on</strong> forms of MNM are ir<strong>on</strong>, vitamin A andiodine deficiency. Together, these affect at least <strong>on</strong>e third of the world’s populati<strong>on</strong>,the majority of whom are in developing countries. Of the three, ir<strong>on</strong> deficiencyis the most prevalent. It is estimated that just over 2 billi<strong>on</strong> people areanaemic, just under 2 billi<strong>on</strong> have inadequate iodine nutriti<strong>on</strong> and 254 milli<strong>on</strong>preschool-aged children are vitamin A deficient (Table 1.1).From a public health viewpoint, MNM is a c<strong>on</strong>cern not just because suchlarge numbers of people are affected, but also because MNM, being a risk factorfor many diseases, can c<strong>on</strong>tribute to high rates of morbidity and even mortality.It has been estimated that micr<strong>on</strong>utrient deficiencies account for about 7.3% ofthe global burden of disease, <strong>with</strong> ir<strong>on</strong> and vitamin A deficiency ranking am<strong>on</strong>gthe 15 leading causes of the global disease burden (4).According to WHO mortality data, around 0.8 milli<strong>on</strong> deaths (1.5% of thetotal) can be attributed to ir<strong>on</strong> deficiency each year, and a similar numberto vitamin A deficiency. In terms of the loss of healthy life, expressed indisability-adjusted life years (DALYs), ir<strong>on</strong>-deficiency anaemia results in25 milli<strong>on</strong> DALYs lost (or 2.4% of the global total), vitamin A deficiencyin 18 milli<strong>on</strong> DALYs lost (or 1.8% of the global total) and iodine deficiencyin 2.5 milli<strong>on</strong> DALYs lost (or 0.2% of the global total) (4).The scale and impact of deficiencies in other micr<strong>on</strong>utrients is much moredifficult to quantify, although it is likely that some forms of MNM, including3
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 1.1Prevalence of the three major micr<strong>on</strong>utrient deficiencies by WHO regi<strong>on</strong>WHO Anaemia a Insufficient Vitamin Aregi<strong>on</strong> (total populati<strong>on</strong>) iodine intake b deficiency c(total(preschool children)populati<strong>on</strong>)No. % of No. % of No. % of(milli<strong>on</strong>s) total (milli<strong>on</strong>s) total (milli<strong>on</strong>s) totalAfrica 244 46 260 43 53 49Americas 141 19 75 10 16 20South-East Asia 779 57 624 40 127 69Europe 84 10 436 57 No data availableEastern 184 45 229 54 16 22MediterraneanWestern Pacific 598 38 365 24 42 27Total 2030 37 1989 35 254 42aBased <strong>on</strong> the proporti<strong>on</strong> of the populati<strong>on</strong> <strong>with</strong> haemoglobin c<strong>on</strong>centrati<strong>on</strong>s below establishedcut-off levels.bBased <strong>on</strong> the proporti<strong>on</strong> of the populati<strong>on</strong> <strong>with</strong> urinary iodine
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEMHowever, c<strong>on</strong>sumpti<strong>on</strong> of a diet that c<strong>on</strong>tains a high proporti<strong>on</strong> of energy-densebut micr<strong>on</strong>utrient-poor processed <strong>food</strong>s can put some populati<strong>on</strong> groups at riskof MNM. Although at present this practice is more comm<strong>on</strong> in industrializedcountries, it is rapidly becoming more prevalent am<strong>on</strong>g countries undergoingsocial and ec<strong>on</strong>omic transiti<strong>on</strong>.Table 1.2 provides an overview of the prevalence, risk factors, and health c<strong>on</strong>sequencesof deficiencies in each of the 15 micr<strong>on</strong>utrients covered in theseeguidelines. For reas<strong>on</strong>s stated above, prevalence estimates are <strong>on</strong>ly provided forir<strong>on</strong> vitamin A and iodine deficiencies. Further informati<strong>on</strong> is available from theWHO Vitamin and Mineral Nutriti<strong>on</strong> Informati<strong>on</strong> System 1 .Up until the 1980s, efforts to alleviate undernutriti<strong>on</strong> in developing countrieswere focused <strong>on</strong> protein–energy malnutriti<strong>on</strong> (PEM). While PEM certainlyremains an important c<strong>on</strong>cern, we have since come to appreciate the significanceof micr<strong>on</strong>utrient malnutriti<strong>on</strong> in terms of its effect <strong>on</strong> human health andfuncti<strong>on</strong>. As a result, the past two decades have seen an increase in activities thatseek to understand and c<strong>on</strong>trol specific micr<strong>on</strong>utrient deficiencies (7). Effortsto c<strong>on</strong>trol iodine deficiency in developing countries, for example, were givennew impetus in the early 1980s when it was recognized that iodine deficiencywas the most comm<strong>on</strong> cause of preventable brain damage and mental retardati<strong>on</strong>in childhood (8,9). There were also reports of increased risks of stillbirthsand low-birth-weight infants in iodine deficient areas (10,11). Importantly, thetechnology to prevent iodine deficiency – salt iodizati<strong>on</strong> – already existed and,moreover, was easy to implement and affordable even by governments <strong>with</strong>limited health budgets. It therefore seemed likely that salt iodizati<strong>on</strong> could be afeasible opti<strong>on</strong> for preventing iodine deficiency <strong>on</strong> a global scale.Similarly, having established that vitamin A status is an important determinantof child survival – in additi<strong>on</strong> to preventing and treating eye disorders, supplementati<strong>on</strong>of vitamin A-deficient children lowers their risk of morbidity(particularly that related to severe diarrhoea), and reduces mortality frommeasles and all-cause mortality (12,13) – measures to c<strong>on</strong>trol vitamin A deficiencyhave been initiated in several world regi<strong>on</strong>s. Reports that ir<strong>on</strong> supplementati<strong>on</strong>of ir<strong>on</strong>-deficient individuals can improve cognitive functi<strong>on</strong>, schoolperformance and work capacity (14,15), and that severe anaemia increases therisk of maternal and child mortality (16), have provided a str<strong>on</strong>g rati<strong>on</strong>ale forir<strong>on</strong> interventi<strong>on</strong>s. Interventi<strong>on</strong> trials have also revealed that zinc supplementati<strong>on</strong>improves the growth of stunted, zinc-deficient children (17), lowers ratesof diarrhoea and pneum<strong>on</strong>ia (the two leading causes of child death), and shortensthe durati<strong>on</strong> of diarrhoeal episodes (18,19).In the wake of such accumulated evidence, the internati<strong>on</strong>al community hasincreasingly come to recognize the public health importance of MNM. In 1990,1See http://www.who.int/nutriti<strong>on</strong>/en5
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 1.2Micr<strong>on</strong>utrient deficiencies: prevalence, risk factors and health c<strong>on</strong>sequencesMicr<strong>on</strong>utrient a Prevalence of deficiency Risk factors Health c<strong>on</strong>sequencesIr<strong>on</strong> There are an estimated 2 billi<strong>on</strong> cases Low intakes of meat/fish/poultry Reduced cognitive performanceof anaemia worldwide and high intakes of cereals and Lower work performance and enduranceIn developing countries, anaemia legumes Impaired iodine and vitamin A metabolismprevalence rates are estimated to be Preterm delivery or low birth Anaemiaabout 50% in pregnant women and weight Increased risk of maternal mortality and childinfants under 2 years, 40% in Pregnancy and adolescence mortality (<strong>with</strong> more severe anaemia)school-aged children and 25–55% in (periods during whichother women and children requirements for ir<strong>on</strong> areIr<strong>on</strong> deficiency is estimated to be especially high)resp<strong>on</strong>sible for around 50% of all Heavy menstrual lossesanaemia cases Parasite infecti<strong>on</strong>s (i.e.There are approximately 1 billi<strong>on</strong> cases hookworm, schistosomiasis,of ir<strong>on</strong>-deficiency anaemia and a ascaris) which cause heavyfurther 1 billi<strong>on</strong> cases of ir<strong>on</strong> blood lossesdeficiency <strong>with</strong>out anaemia worldwide Malaria (causes anaemia not ir<strong>on</strong>deficiency)Low intakes of vitamin C(ascorbic acid)Allergy to cow’s milkVitamin A An estimated 254 milli<strong>on</strong> preschool Low intakes of dairy products, Increased risk of mortality in children andchildren are vitamin A deficient eggs and β-carotene from pregnant womenfruits and vegetables Night blindness, xerophthalmiaPresence of helminth infecti<strong>on</strong>,ascaris6
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEMIodine An estimated 2 billi<strong>on</strong> people have Residence in areas <strong>with</strong> low Birth defectsinadequate iodine nutriti<strong>on</strong> and levels of iodine in soil and Increased risk of stillbirth and infanttherefore are at risk of iodine water mortalitydeficiency disorders Living in high altitude regi<strong>on</strong>s, Cognitive and neurological impairmentriver plains or far from the sea including cretinismC<strong>on</strong>sumpti<strong>on</strong> of n<strong>on</strong>-detoxified Impaired cognitive functi<strong>on</strong>cassava HypothyroidismGoitreZinc Insufficient data, but prevalence of Low intakes of animal products N<strong>on</strong>-specific if marginal deficiencydeficiency is likely to be moderate to High phytate intakes Possibly poor pregnancy outcomeshigh in developing countries, Malabsorpti<strong>on</strong> and infecti<strong>on</strong> Impaired growth (stunting)especially those in Africa, South-East <strong>with</strong> intestinal parasites Decreased resistance to infectious diseasesAsia and the Western Pacific Diarrhoea, especially persistent Severe deficiency results in dermatitis,Genetic disorders retarded growth, diarrhoea, mentaldisturbance, delayed sexual maturati<strong>on</strong>and/or recurrent infecti<strong>on</strong>sFolate Insufficient data Low intakes of fruits and Megaloblastic anaemia(vitamin B9) vegetables, legumes and Risk factor for:dairy products — neural tube defects and other birthMalabsorpti<strong>on</strong> and intestinal defects (oro-facial clefts, heart defects)parasites infecti<strong>on</strong>s (e.g. and adverse pregnancy outcomes;Giardia Lamblia) — elevated plasma homocysteine;Genetic disorder of folic acid — heart disease and strokemetabolism — impaired cognitive functi<strong>on</strong>— depressi<strong>on</strong>7
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 1.2Micr<strong>on</strong>utrient deficiencies: prevalence, risk factors and health c<strong>on</strong>sequences (C<strong>on</strong>tinued)Micr<strong>on</strong>utrient a Prevalence of deficiency Risk factors Health c<strong>on</strong>sequencesVitamin B12 Insufficient data Low intakes of animal products Megaloblastic anaemia(cobalamin) Malabsorpti<strong>on</strong> from <strong>food</strong> due to Severe deficiency can cause developmentalgastric atrophy induced by delays, poor neurobehavioral performanceHelicobacter pylori, or and growth in infants and children, nervebacterial overgrowth demyelinati<strong>on</strong> and neurologicalGenetic disorder of vitamin B12 dysfuncti<strong>on</strong>metabolism Risk factor for:— neural tube defects;— elevated plasma homocysteine;— impaired cognitive functi<strong>on</strong>Vitamin B1 Insufficient data <strong>on</strong> marginal deficiency High c<strong>on</strong>sumpti<strong>on</strong> of refined Beriberi presents in two forms:(thiamine) Severe deficiency (beriberi) is reported rice and cereals — a cardiac form <strong>with</strong> risk of heart failurein parts of Japan and north-east Low intakes of animal and dairy (predominant in ne<strong>on</strong>ates)Thailand products, and legumes — a neurological form <strong>with</strong> chr<strong>on</strong>icRegularly reported in famine situati<strong>on</strong>s C<strong>on</strong>sumpti<strong>on</strong> of thiaminase peripheral neuropathy (loss ofand am<strong>on</strong>g displaced populati<strong>on</strong>s (found in raw fish) sensati<strong>on</strong> and reflexes)Breastfeeding (from deficient Wernicke-Korsakov syndrome (usually inmothers) alcoholics) <strong>with</strong> c<strong>on</strong>fusi<strong>on</strong>, lack ofChr<strong>on</strong>ic alcoholism coordinati<strong>on</strong> and paralysisGenetic disorder of thiaminemetabolismVitamin B2 Insufficient data, but some evidence Low intakes of animal and dairy Symptoms are n<strong>on</strong>-specific and can include(riboflavin) that it might be very comm<strong>on</strong> in products fatigue, eye changes and in more severedeveloping countries Chr<strong>on</strong>ic alcoholism cases, dermatitis (stomatitis, cheilosis),brain dysfuncti<strong>on</strong> and microcytic anaemiaImpaired ir<strong>on</strong> absorpti<strong>on</strong> and utilizati<strong>on</strong>8
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEMVitamin B 3 Insufficient data <strong>on</strong> marginal deficiency Low intakes of animal and dairy Severe deficiency results in pellagra, which(niacin) Severe deficiency (pellagra) still products is characterized by:comm<strong>on</strong> in Africa, China and India High c<strong>on</strong>sumpti<strong>on</strong> of refined — dermatitis (symmetrical pigmented rashand recently reported am<strong>on</strong>g cereals <strong>on</strong> skin areas exposed to sunlight);displaced populati<strong>on</strong>s (south-eastern Maize-based diets (not lime — digestive mucosa disorders (diarrhoeaAfrica) and in famine situati<strong>on</strong>s treated) and vomiting);— neurological symptoms, depressi<strong>on</strong>and loss of memoryVitamin B6 Insufficient data, but recent reports from Low intakes of animal products Symptoms are n<strong>on</strong>-specific and may include:Egypt and Ind<strong>on</strong>esia suggest High c<strong>on</strong>sumpti<strong>on</strong> of refined — neurological disorders <strong>with</strong>deficiency is likely to be widespread cereals c<strong>on</strong>vulsi<strong>on</strong>s;in developing countries Chr<strong>on</strong>ic alcoholism — dermatitis (stomatitis and cheilosis)Rather uncomm<strong>on</strong> in isolati<strong>on</strong>, being Anaemia (possibly)typically associated <strong>with</strong> deficiencies Deficiency is a risk factor for elevatedin the other B vitamins plasma homocysteineVitamin C Insufficient data <strong>on</strong> moderate Low intakes of fresh vitamin Severe deficiency results in scurvy <strong>with</strong>(ascorbic acid) deficiencies C-rich fruits and vegetables haemorrhagic syndrome (i.e. bleedingSevere deficiency (scurvy) regularly Prol<strong>on</strong>ged cooking gums, joint and muscle pain, peripheralreported in famine situati<strong>on</strong>s (e.g. oedema)east Africa) and am<strong>on</strong>g displaced Anaemiapeople dependent <strong>on</strong> <strong>food</strong> aid forl<strong>on</strong>g periods (e.g. east Africa, Nepal)Vitamin D Insufficient data, but likely to be Low exposure to ultra-violet Severe forms result in rickets in children andcomm<strong>on</strong> in both industrialized and radiati<strong>on</strong> from the sun osteomalacia in adultsdeveloping countries Wearing excess clothingHigher at more northerly and southerly Having darkly pigmented skinlatitudes where daylight hours arelimited during the winter m<strong>on</strong>ths9
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 1.2Micr<strong>on</strong>utrient deficiencies: prevalence, risk factors and health c<strong>on</strong>sequences (C<strong>on</strong>tinued)Micr<strong>on</strong>utrient a Prevalence of deficiency Risk factors Health c<strong>on</strong>sequencesCalcium Insufficient data, but low intakes very Low intakes of dairy products Decreased b<strong>on</strong>e mineralizati<strong>on</strong>comm<strong>on</strong> Increased risk of osteoporosis in adultsIncreased risk of rickets in childrenSelenium Insufficient data <strong>on</strong> moderate deficiency Residing in low selenium Severe deficiency presents as:Severe deficiency reported in some envir<strong>on</strong>ments — cardiomyopathy (Keshan disease), orregi<strong>on</strong>s of China, Japan, Korea, New Low intakes of animal products — osteoarthropathy in children (Kaschin-Zealand, Scandinavia and Siberia Some evidence that symptoms Beck disease)are not due to selenium Increased risk of cancer and cardiovasculardeficiency al<strong>on</strong>e, but also to diseasethe presence of the cocksackie Exacerbati<strong>on</strong> of thyroid dysfuncti<strong>on</strong> causedvirus (Keshan disease) or by iodine deficiencymycotoxins (Kaschin-Beckdisease)Fluoride NA Residing in areas <strong>with</strong> low fluoride Increased risk of dental decaylevels in waterNA, not applicable.aMicr<strong>on</strong>utrients are listed in order of their public health significance.Sources: adapted from references (1–3,5,6).10
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEMthe World Health Assembly passed a landmark resoluti<strong>on</strong> urging acti<strong>on</strong> byMember States “to prevent and c<strong>on</strong>trol iodine deficiency disorders” (20). Laterthat year, at the World Summit for Children, the world’s leaders endorsed the“virtual eliminati<strong>on</strong> of iodine and vitamin A deficiency and a reducti<strong>on</strong> of theprevalence of ir<strong>on</strong>-deficiency anaemia in women by <strong>on</strong>e third”.These goals havebeen reiterated at a number of subsequent internati<strong>on</strong>al fora, including theM<strong>on</strong>treal c<strong>on</strong>ference <strong>on</strong> Ending Hidden Hunger in 1991, the 1992 FAO/WHOInternati<strong>on</strong>al C<strong>on</strong>ference <strong>on</strong> Nutriti<strong>on</strong> held in Rome, the 1993 World HealthAssembly held in Geneva, and the Special Sessi<strong>on</strong> <strong>on</strong> Children of the UnitedNati<strong>on</strong>s General Assembly, which was held in New York in 2002.There has beenremarkable degree of c<strong>on</strong>sensus and support for MNM c<strong>on</strong>trol between governments,United Nati<strong>on</strong>s agencies, multilateral and bilateral agencies, academicand research instituti<strong>on</strong>s, n<strong>on</strong>governmental organizati<strong>on</strong>s (NGOs) and d<strong>on</strong>orfoundati<strong>on</strong>s. More recently, following recogniti<strong>on</strong> of the essential role played byindustry – in particular, the salt, <strong>food</strong> and drug industries – str<strong>on</strong>ger links <strong>with</strong>the private sector have been forged. This is reflected by the implementati<strong>on</strong> ofseveral public–private coaliti<strong>on</strong>s aimed at addressing the main micr<strong>on</strong>utrientdeficiencies, which include the Global Alliance for Improved Nutriti<strong>on</strong> 1 and TheGlobal Network for Sustained Eliminati<strong>on</strong> of Iodine Deficiency 2 .1.2 Strategies for the c<strong>on</strong>trol of micr<strong>on</strong>utrient malnutriti<strong>on</strong>The c<strong>on</strong>trol of vitamin and mineral deficiencies is an essential part of the overalleffort to fight hunger and malnutriti<strong>on</strong>. Countries need to adopt and support acomprehensive approach that addresses the causes of malnutriti<strong>on</strong> and the oftenassociated “hidden hunger” which rest intinsic to in poverty and unsustainablelivelihoods. Acti<strong>on</strong>s that promote an increase in the supply, access, c<strong>on</strong>sumpti<strong>on</strong>and utilizati<strong>on</strong> of an adequate quantity, quality and variety of <strong>food</strong>s for allpopulati<strong>on</strong>s groups should be supported. The aim is for all people to be able toobtain from their diet all the energy, macro- and micr<strong>on</strong>utrients they need toenjoy a healthy and productive life.Policy and programme resp<strong>on</strong>ses include <strong>food</strong>-based strategies such asdietary diversificati<strong>on</strong> and <strong>food</strong> fortificati<strong>on</strong>, as well as nutriti<strong>on</strong> educati<strong>on</strong>,public health and <strong>food</strong> safety measures, and finally supplementati<strong>on</strong>. Theseapproaches should be regarded as complementary, <strong>with</strong> their relative importancedepending <strong>on</strong> local c<strong>on</strong>diti<strong>on</strong>s and the specific mix of local needs.Of the three opti<strong>on</strong>s that are aimed at increasing the intake of micr<strong>on</strong>utrients,programmes that deliver micr<strong>on</strong>utrient supplements often provide the fastestimprovement in the micr<strong>on</strong>utrient status of individuals or targeted populati<strong>on</strong>12See http://www.gainhealth.org.See http://www.iodinenetwork.net.11
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSgroups. Food fortificati<strong>on</strong> tends to have a less immediate but nevertheless amuch wider and more sustained impact. Although increasing dietary diversityis generally regarded as the most desirable and sustainable opti<strong>on</strong>, it takes thel<strong>on</strong>gest to implement.1.2.1 Increasing the diversity of <strong>food</strong>s c<strong>on</strong>sumedIncreasing dietary diversity means increasing both the quantity and the range ofmicr<strong>on</strong>utrient-rich <strong>food</strong>s c<strong>on</strong>sumed. In practice, this requires the implementati<strong>on</strong>of programmes that improve the availability and c<strong>on</strong>sumpti<strong>on</strong> of, and accessto, different types of micr<strong>on</strong>utrient-rich <strong>food</strong>s (such as animal products, fruitsand vegetables) in adequate quantities, especially am<strong>on</strong>g those who at risk for,or vulnerable to, MNM. In poorer communities, attenti<strong>on</strong> also needs to be paidto ensuring that dietary intakes of oils and fats are adequate for enhancing theabsorpti<strong>on</strong> of the limited supplies of micr<strong>on</strong>utrients.Increasing dietary diversity is the preferred way of improving the nutriti<strong>on</strong> ofa populati<strong>on</strong> because it has the potential to improve the intake of many <strong>food</strong>c<strong>on</strong>stituents – not just micr<strong>on</strong>utrients – simultaneously. Ongoing research suggeststhat micr<strong>on</strong>utrient-rich <strong>food</strong>s also provide a range of antioxidants andprobiotic substances that are important for protecti<strong>on</strong> against selected n<strong>on</strong>communicablediseases and for enhancing immune functi<strong>on</strong>. However, as astrategy for combating MNM, increasing dietary diversity is not <strong>with</strong>out its limitati<strong>on</strong>s,the main <strong>on</strong>e being the need for behaviour change and for educati<strong>on</strong>about how certain <strong>food</strong>s provide essential micr<strong>on</strong>utrients and other nutritivesubstances. A lack of resources for producing and purchasing higher quality<strong>food</strong>s can sometimes present a barrier to achieving greater dietary diversity,especially in the case of poorer populati<strong>on</strong>s. The importance of animal source<strong>food</strong>s for dietary quality is increasingly being recognized, and innovativeapproaches to increase their producti<strong>on</strong> and c<strong>on</strong>sumpti<strong>on</strong> in poorer regi<strong>on</strong>s ofthe world are currently being explored (21). Efforts are also underway to helppoorer communities identify, domesticate and cultivate traditi<strong>on</strong>al and wildmicr<strong>on</strong>utrient-rich <strong>food</strong>s as a simple and affordable means of satisfyingmicr<strong>on</strong>utrient needs (22–24).For infants, ensuring a diet of breast milk is an effective way of preventingmicr<strong>on</strong>utrient deficiencies. In much of the developing world, breast milk is themain source of micr<strong>on</strong>utrients during the first year of life (<strong>with</strong> the excepti<strong>on</strong>of ir<strong>on</strong>). Exclusive breastfeeding for the first 6 m<strong>on</strong>ths of life and c<strong>on</strong>tinuati<strong>on</strong>into the sec<strong>on</strong>d year should thus be promoted. Moreover, all lactating womenshould be encouraged to c<strong>on</strong>sume a healthful and varied diet so that adequatelevels of micr<strong>on</strong>utrients are secreted in their milk. After the age of 6 m<strong>on</strong>ths, itis important that the complementary <strong>food</strong>s provided to breast-fed infants are asdiverse and as rich in micr<strong>on</strong>utrients as possible.12
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEM1.2.2 Food fortificati<strong>on</strong>Food fortificati<strong>on</strong> refers to the additi<strong>on</strong> of micr<strong>on</strong>utrients to processed <strong>food</strong>s.In many situati<strong>on</strong>s, this strategy can lead to relatively rapid improvementsin the micr<strong>on</strong>utrient status of a populati<strong>on</strong>, and at a very reas<strong>on</strong>able cost,especially if advantage can be taken of existing technology and local distributi<strong>on</strong>networks. Since the benefits are potentially large, <strong>food</strong> fortificati<strong>on</strong>can be a very cost-effective public health interventi<strong>on</strong>. However, an obviousrequirement is that the fortified <strong>food</strong>(s) needs to be c<strong>on</strong>sumed in adequateamounts by a large proporti<strong>on</strong> of the target individuals in a populati<strong>on</strong>. It isalso necessary to have access to, and to use, fortificants that are well absorbedyet do not affect the sensory properties of <strong>food</strong>s. In most cases, it is preferableto use <strong>food</strong> vehicles that are centrally processed, and to have the support of the<strong>food</strong> industry.Fortificati<strong>on</strong> of <strong>food</strong> <strong>with</strong> micr<strong>on</strong>utrients is a valid technology for reducingmicr<strong>on</strong>utrient malnutriti<strong>on</strong> as part of a <strong>food</strong>-based approach when and whereexisting <strong>food</strong> supplies and limited access fail to provide adequate levels of therespective nutrients in the diet. In such cases, <strong>food</strong> fortificati<strong>on</strong> reinforces andsupports <strong>on</strong>going nutriti<strong>on</strong> improvement programmes and should be regardedas part of a broader, integrated approach to prevent MNM, thereby complementingother approaches to improve micr<strong>on</strong>utrient status.1.2.3 Supplementati<strong>on</strong>Supplementati<strong>on</strong> is the term used to describe the provisi<strong>on</strong> of relatively largedoses of micr<strong>on</strong>utrients, usually in the form of pills, capsules or syrups. It hasthe advantage of being capable of supplying an optimal amount of a specificnutrient or nutrients, in a highly absorbable form, and is often the fastest wayto c<strong>on</strong>trol deficiency in individuals or populati<strong>on</strong> groups that have been identifiedas being deficient.In developing countries, supplementati<strong>on</strong> programmes have been widely usedto provide ir<strong>on</strong> and folic acid to pregnant women, and vitamin A to infants, childrenunder 5 years of age and postpartum women. Because a single high-dosevitamin A supplement improves vitamin A stores for about 4–6 m<strong>on</strong>ths, supplementati<strong>on</strong>two or three times a year is usually adequate. However, in the caseof the more water-soluble vitamins and minerals, supplements need to be c<strong>on</strong>sumedmore frequently. Supplementati<strong>on</strong> usually requires the procurement andpurchase of micr<strong>on</strong>utrients in a relatively expensive pre-packaged form, aneffective distributi<strong>on</strong> system and a high degree of c<strong>on</strong>sumer compliance (especiallyif supplements need to be c<strong>on</strong>sumed <strong>on</strong> a l<strong>on</strong>g-term basis). A lack of suppliesand poor compliance are c<strong>on</strong>sistently reported by many supplementati<strong>on</strong>programme managers as being the main barriers to success.13
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS1.2.4 Public health measuresIn additi<strong>on</strong> to the specific interventi<strong>on</strong>s outlined above, public health measuresof a more general nature are often required to help prevent and correct MNM,because MNM is often associated <strong>with</strong> poor overall nutriti<strong>on</strong>al status and <strong>with</strong>a high prevalence of infecti<strong>on</strong>. Such measures include infecti<strong>on</strong> c<strong>on</strong>trol (e.g.immunizati<strong>on</strong>, malaria and parasite c<strong>on</strong>trol), and improvement of water and sanitati<strong>on</strong>.Other factors, such as the quality of child care and maternal educati<strong>on</strong>,also need to be taken into c<strong>on</strong>siderati<strong>on</strong> when developing public health resp<strong>on</strong>sesto MNM.1.3 Food fortificati<strong>on</strong> in practiceFood fortificati<strong>on</strong> has a l<strong>on</strong>g history of use in industrialized countries for thesuccessful c<strong>on</strong>trol of deficiencies of vitamins A and D, several B vitamins (thiamine,riboflavin and niacin), iodine and ir<strong>on</strong>. Salt iodizati<strong>on</strong> was introduced inthe early 1920s in both Switzerland (25) and the United States of America (26)and has since expanded progressively all over the world to the extent that iodizedsalt is now used in most countries. From the early 1940s <strong>on</strong>wards, the fortificati<strong>on</strong>of cereal products <strong>with</strong> thiamine, riboflavin and niacin became comm<strong>on</strong>practice. Margarine was fortified <strong>with</strong> vitamin A in Denmark and milk <strong>with</strong>vitamin D in the United States. Foods for young children were fortified <strong>with</strong>ir<strong>on</strong>, a practice which has substantially reduced the risk of ir<strong>on</strong>-deficiencyanaemia in this age group. In more recent years, folic acid fortificati<strong>on</strong> of wheathas become widespread in the Americas, a strategy adopted by Canada and theUnited States and about 20 Latin American countries.In the less industrialized countries, fortificati<strong>on</strong> has become an increasinglyattractive opti<strong>on</strong> in recent years, so much so that planned programmes havemoved forward to the implementati<strong>on</strong> phase more rapidly than previouslythought possible. Given the success of the relatively l<strong>on</strong>g-running programmeto fortify sugar <strong>with</strong> vitamin A in Central America, where the prevalence ofvitamin A deficiency has been reduced c<strong>on</strong>siderably, similar initiatives are beingattempted in other world regi<strong>on</strong>s. Currently, the first sugar fortificati<strong>on</strong> experiencein sub-Saharan Africa is taking place in Zambia, and if successful will beemulated elsewhere. Darnt<strong>on</strong>-Hill and Nalubola (27) have identified at least 27developing countries that could benefit from programmes to fortify <strong>on</strong>e or more<strong>food</strong>s.Despite apparent past successes, to date, very few fortificati<strong>on</strong> programmeshave formally evaluated their impact <strong>on</strong> nutriti<strong>on</strong>al status. However, <strong>with</strong>out aspecific evaluati<strong>on</strong> comp<strong>on</strong>ent, <strong>on</strong>ce a fortificati<strong>on</strong> programme has been initiated,it is difficult to know whether subsequent improvements in the nutriti<strong>on</strong>alstatus of a populati<strong>on</strong> are due to the interventi<strong>on</strong> or to other changes, such as,improvements in socioec<strong>on</strong>omic status or in public health provisi<strong>on</strong>, that14
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEMoccurred over the same period of time. Evidence that <strong>food</strong> fortificati<strong>on</strong> programmesdo indeed improve nutriti<strong>on</strong>al status has therefore tended to comefrom either efficacy trials and/or reports of programme effectiveness. Efficacytrials, i.e. trials c<strong>on</strong>ducted in c<strong>on</strong>trolled feeding situati<strong>on</strong>s, are relatively numerousand have usefully documented the impact of fortified <strong>food</strong>s <strong>on</strong> nutriti<strong>on</strong>alstatus and other outcomes. Evidence of programme effectiveness, which isobtained by assessing changes in nutriti<strong>on</strong>al status and other outcomes <strong>on</strong>cea programme has been implemented, is less widely available. Of the feweffectiveness studies that have been c<strong>on</strong>ducted, even fewer included a n<strong>on</strong>interventi<strong>on</strong>c<strong>on</strong>trol group, an omissi<strong>on</strong> that weakens the evidence that can beobtained from studies of this type.1.3.1 Efficacy trialsAs indicated above, efficacy trials evaluate the impact of a test interventi<strong>on</strong> underideal circumstances. In the case of <strong>food</strong> fortificati<strong>on</strong>, this typically involves alltest subjects c<strong>on</strong>suming a known amount of the fortified <strong>food</strong>. In the majorityof efficacy trials c<strong>on</strong>ducted to date, fortified <strong>food</strong>s have been shown to improvemicr<strong>on</strong>utrient status. Selected examples, involving a range of micr<strong>on</strong>utrients, arebriefly described below. The general principles of programme impact evaluati<strong>on</strong>,including the design of efficacy trials, are discussed in greater detail inChapter 8 of these guidelines.1.3.1.1 Ir<strong>on</strong> fortificati<strong>on</strong>In Viet Nam, 6-m<strong>on</strong>th efficacy trials have established that fortificati<strong>on</strong> of fishsauce <strong>with</strong> ir<strong>on</strong> can significantly improve ir<strong>on</strong> status and reduce anaemia andir<strong>on</strong> deficiency (28). The subjects were n<strong>on</strong>-pregnant anaemic female factoryworkers who c<strong>on</strong>sumed 10 ml per day of a sauce that was fortified <strong>with</strong> 100 mgir<strong>on</strong> (as NaFeEDTA) per 100 ml. Figure 1.1 illustrates the effect of the interventi<strong>on</strong><strong>on</strong> ir<strong>on</strong> deficiency and ir<strong>on</strong>-deficiency anaemia; both were significantlyreduced after 6 m<strong>on</strong>ths in the group receiving the fortified sauce relative to theplacebo c<strong>on</strong>trol group.In China, a series of studies have been c<strong>on</strong>ducted to assess the efficacy, effectivenessand feasibility of fortifying soy sauce <strong>with</strong> ir<strong>on</strong> (in the form ofNaFeEDTA). Daily c<strong>on</strong>sumpti<strong>on</strong> of 5 mg or 20 mg ir<strong>on</strong> in the fortified saucewas reported to be very effective in the treatment of ir<strong>on</strong>-deficiency anaemia inchildren; positive effects were seen <strong>with</strong>in 3 m<strong>on</strong>ths of the start of the interventi<strong>on</strong>(J. Chen, cited in (29). In a double-blind placebo-c<strong>on</strong>trolled effectivenesstrial of the ir<strong>on</strong>-fortified sauce, involving about 10 000 children and women,a reducti<strong>on</strong> in the prevalence of anaemia was observed <strong>with</strong>in 6 m<strong>on</strong>ths (seealso secti<strong>on</strong> 1.3.2.2).15
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSFIGURE 1.1Effect of ir<strong>on</strong> fortificati<strong>on</strong> of fish sauce <strong>on</strong> ir<strong>on</strong> status of n<strong>on</strong>-pregnantanaemic female Vietnamese factory workersPrevalence (%)Ir<strong>on</strong> deficiency8070605040*3020100t 0 t 3 t 6TimePrevalence (%)Ir<strong>on</strong> deficiency anaemia80706050403020**100t 0 t 3 t 6TimePrevalence of ir<strong>on</strong> deficiency and ir<strong>on</strong> deficiency anaemia at baseline, and after 3and 6 m<strong>on</strong>ths of interventi<strong>on</strong> in the ir<strong>on</strong> interventi<strong>on</strong> group (10 mg ir<strong>on</strong>/day inNaFeEDTA-fortified fish sauce (n = 64)) and the c<strong>on</strong>trol group (n = 72) in anaemicVietnamese women.Source: reproduced from reference (28), <strong>with</strong> the permissi<strong>on</strong> of the publishers.In an ir<strong>on</strong>-deficient Indian populati<strong>on</strong> in South Africa, fortificati<strong>on</strong> of currypowder <strong>with</strong> NaFeEDTA produced significant improvements in blood haemoglobin,ferritin levels and ir<strong>on</strong> stores in women, and in ferritin levels in men (30).During the 2-year study, the prevalence of ir<strong>on</strong>-deficiency anaemia in womenfell from 22% to just 5%.Regrettably, well-designed trials of the impact of ir<strong>on</strong> fortificati<strong>on</strong> of flour arelacking at the present time.1.3.1.2 Vitamin A fortificati<strong>on</strong>Trials c<strong>on</strong>ducted in the Philippines have revealed that fortificati<strong>on</strong> ofm<strong>on</strong>osodium glutamate <strong>with</strong> vitamin A produces positive effects <strong>on</strong> child mortality,and improved growth and haemoglobin levels in children (31). Laterstudies <strong>with</strong> preschool-aged children, who c<strong>on</strong>sumed 27 g of vitamin A-fortifiedmargarine per day for a period of 6 m<strong>on</strong>ths, reported a reducti<strong>on</strong> in the prevalenceof low serum retinol c<strong>on</strong>centrati<strong>on</strong>s from 26% to 10% (32). Wheatflour fortified <strong>with</strong> vitamin A and fed as buns to Filipino schoolchildren for 30weeks had the effect of halving the number that had low liver stores of thevitamin (33).1.3.1.3 Multiple fortificati<strong>on</strong>A number of trials have evaluated the efficacy of specially-formulated <strong>food</strong>s andbeverages as vehicles for multiple fortificati<strong>on</strong>. In South Africa, for example, for-16
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEMtificati<strong>on</strong> of biscuits <strong>with</strong> ir<strong>on</strong>, β-carotene and iodine improved the status of allof these nutrients in schoolchildren (34). Vitamin A and ir<strong>on</strong> status deterioratedduring the l<strong>on</strong>g school holidays when the biscuits were not fed. Fortificati<strong>on</strong>of a flavoured beverage <strong>with</strong> 10 micr<strong>on</strong>utrients increased serum retinol andreduced ir<strong>on</strong> deficiency in Tanzanian schoolchildren, and also improvedtheir growth rates (35). Similarly, in Botswana, regular c<strong>on</strong>sumpti<strong>on</strong> of a 12-micr<strong>on</strong>utrient enriched beverage by school-aged children increased their weightgain and mid-upper arm circumference, and improved their ir<strong>on</strong>, folate,riboflavin and zinc status (36).1.3.2 Effectiveness evaluati<strong>on</strong>sThe aim of an effectiveness evaluati<strong>on</strong> is to assess the impact of an interventi<strong>on</strong>or programme in actual practice, as opposed to under c<strong>on</strong>trolled c<strong>on</strong>diti<strong>on</strong>s.Because of factors such as the lack of c<strong>on</strong>sumpti<strong>on</strong> of the fortified <strong>food</strong>, themagnitude of the impact of an interventi<strong>on</strong> is likely to be less than that in anefficacy trial (see also Chapter 8: M<strong>on</strong>itoring and evaluati<strong>on</strong>).1.3.2.1 Iodine fortificati<strong>on</strong>Numerous studies, particularly from the developed world, have clearly establishedthat salt iodizati<strong>on</strong> is an effective means of c<strong>on</strong>trolling iodine deficiency.In the United States, large-scale iodizati<strong>on</strong> of salt in Michigan reduced the goitrerate from about 40% to below 10% (26). In the early 20th century almost allSwiss schoolchildren had goitre and 0.5% of the populati<strong>on</strong> had cretinism.Whensalt iodizati<strong>on</strong> was introduced in 1922, the prevalence of goitre and deaf mutismin children dropped dramatically. Since then, a sustained salt iodizati<strong>on</strong> programmehas ensured an adequate iodine status am<strong>on</strong>g the whole Swiss populati<strong>on</strong>(25). Despite such c<strong>on</strong>vincing evidence in support of salt iodizati<strong>on</strong>, in asrecently as 2003, it was estimated that 54 countries still have inadequate iodinenutriti<strong>on</strong> (i.e. median urinary iodine < 100 µg/l) (2).1.3.2.2 Ir<strong>on</strong> fortificati<strong>on</strong>The effectiveness of ir<strong>on</strong> fortificati<strong>on</strong> has been dem<strong>on</strong>strated in several worldregi<strong>on</strong>s. Ir<strong>on</strong> fortificati<strong>on</strong> of infant formulas has been associated <strong>with</strong> a fall inthe prevalence of anaemia in children aged under 5 years in the United States(37,38). In Venezuela, wheat and maize flours have been fortified <strong>with</strong> ir<strong>on</strong> (asa mixture of ferrous fumarate and elemental ir<strong>on</strong>), vitamin A and various B vitaminssince 1993. A comparis<strong>on</strong> of the prevalence of ir<strong>on</strong> deficiency and anaemiapre- and post-interventi<strong>on</strong> showed a significant reducti<strong>on</strong> in the prevalence ofthese c<strong>on</strong>diti<strong>on</strong>s in children (39). Fortificati<strong>on</strong> of milk <strong>with</strong> ir<strong>on</strong> and vitamin C(ascorbic acid) in Chile produced a rapid reducti<strong>on</strong> in the prevalence of ir<strong>on</strong>17
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSdeficiency in infants and young children (40,41). The effectiveness of the fortificati<strong>on</strong>of soy sauce <strong>with</strong> ir<strong>on</strong> is currently being evaluated in a populati<strong>on</strong> of10 000 Chinese women and children <strong>with</strong> a high risk of anaemia. Preliminaryresults of the 2-year double-blind placebo-c<strong>on</strong>trolled study have shown a reducti<strong>on</strong>in anaemia prevalence rates for all age groups after the first 6 m<strong>on</strong>ths(J. Chen, cited in (29).Unfortunately, very few other ir<strong>on</strong> fortificati<strong>on</strong> programmes have been evaluated.Informati<strong>on</strong> about the efficacy and effectiveness of flour fortificati<strong>on</strong> inparticular is urgently needed (42).1.3.2.3 Combined ir<strong>on</strong> and iodine fortificati<strong>on</strong>A randomized, double-blind effectiveness trial in Moroccan schoolchildren(n = 367) has dem<strong>on</strong>strated that the dual fortificati<strong>on</strong> of salt <strong>with</strong> ir<strong>on</strong> and iodinecan improve both ir<strong>on</strong> and iodine status (43). Results of the 40-week trial, inwhich salt was fortified <strong>with</strong> ir<strong>on</strong> at a level of 1 mg Fe/g salt (as ferrous sulfatemicroencapsulated <strong>with</strong> partially hydrogenated vegetable oil) are summarized inFigure 1.2. In additi<strong>on</strong> to improved ir<strong>on</strong> status, by the end of the trial the ir<strong>on</strong>fortifiedgroup had significantly lower thyroid volumes. Because ir<strong>on</strong> is requiredfor thyroxine synthesis, ir<strong>on</strong> deficiency reduces the efficacy of iodine prophylaxis.Thus, by supplying both iodine and ir<strong>on</strong>, the impact of iodine fortificati<strong>on</strong>is maximized.FIGURE 1.2Effect of dual-fortified salt (ir<strong>on</strong> and iodine) <strong>on</strong> the ir<strong>on</strong> status of MoroccanschoolchildrenPrevalence (%)40353025201510500 20 40Time (wk)IDA, IS groupID <strong>with</strong>out anaemia, IS groupIDA, DFS groupID <strong>with</strong>out anaemia, DFS groupThe probability of ir<strong>on</strong> deficiency anaemia (IDA) and ir<strong>on</strong> deficiency <strong>with</strong>out anaemia(ID) was significantly less in 6–15 year-old children receiving dual-fortified salt (DFS)c<strong>on</strong>taining both ir<strong>on</strong> and iodine (n = 183) than in those receiving iodized salt (IS) (n= 184). For both IDA and ID <strong>with</strong>out anaemia, the difference between the IS and DFSgroups increased significantly <strong>with</strong> time (P < 0.01).Source: reproduced from reference (44).18
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEM1.3.2.4 Vitamin A fortificati<strong>on</strong>Fortificati<strong>on</strong> of sugar <strong>with</strong> vitamin A is a strategy that has been used extensivelythroughout Central America. Starting in Guatemala in 1974, and extending toother countries in the regi<strong>on</strong> in subsequent years, the effect of this programmehas been to reduce the prevalence of low serum retinol values – from 27% in1965 to 9% in 1977 (45,46). There is also evidence to suggest that sugar fortificati<strong>on</strong>substantially increases the c<strong>on</strong>centrati<strong>on</strong> of vitamin A in breast milk(47). When the programme was temporarily disc<strong>on</strong>tinued in parts of the regi<strong>on</strong>,the prevalence of low serum retinol again increased. Vitamin A fortificati<strong>on</strong> ofsugar is, however, still <strong>on</strong>going in Guatemala.1.3.2.5 Folic acid fortificati<strong>on</strong>The introducti<strong>on</strong> of the mandatory fortificati<strong>on</strong> of wheat flour <strong>with</strong> folic acid inthe United States in 1998 was accompanied by a significant reducti<strong>on</strong> in theprevalence of neural tube defects (48) and in plasma levels of homocysteine.(Elevated plasma homocysteine has been identified as a risk factor for cardiovasculardisease and other health problems (49). Even though these outcomesmay have been due to other factors, there was certainly an increase in folateintakes (50) and an improvement in folate status (49) am<strong>on</strong>g the populati<strong>on</strong> inthe period immediately following the implementati<strong>on</strong> of the new legislati<strong>on</strong>.Similar improvements in folate status have been seen after the commencementof folic acid fortificati<strong>on</strong> of wheat flour in Canada (51) (see Figure 1.3).FIGURE 1.3Effect of flour fortificati<strong>on</strong> <strong>with</strong> folic acid <strong>on</strong> the folate status of elderlyCanadian women30Serum folate (nmol/l)25201510501996 1997 1998 1999 2000Serum folate c<strong>on</strong>centrati<strong>on</strong>s in a cross-secti<strong>on</strong> of 15 664 Canadian women aged 65years and older in relati<strong>on</strong> to the introducti<strong>on</strong> of flour fortificati<strong>on</strong> in mid-1997. Dataare presented as mean values (solid line) <strong>with</strong> 95% c<strong>on</strong>fidence limits (dotted lines)Source: reproduced from reference (53), <strong>with</strong> the permissi<strong>on</strong> of the publishers.Year19
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSLikewise, in Chile, a nati<strong>on</strong>al programme of flour fortificati<strong>on</strong> <strong>with</strong> folic acidincreased serum folate and reduced serum homocysteine in a group of elderlypeople (52).1.3.2.6 Fortificati<strong>on</strong> <strong>with</strong> other B vitaminsBeriberi, riboflavin deficiency, pellagra and anaemia were relatively widespreadpublic health problems during the 1930s in several countries, including theUnited States. In an attempt to reduce the prevalence of these c<strong>on</strong>diti<strong>on</strong>s, adecisi<strong>on</strong> was taken to add thiamine, riboflavin, niacin and ir<strong>on</strong> to wheat flour.With the implementati<strong>on</strong> of fortificati<strong>on</strong> programmes for these micr<strong>on</strong>utrientsduring the early 1940s in the United States and in some European countries,these deficiencies largely disappeared (54). While it can be argued thatother factors – such as improved dietary diversity – also played a role, enrichedflour c<strong>on</strong>tinues to make an important c<strong>on</strong>tributi<strong>on</strong> to meeting recommendednutrient intakes for the B-complex vitamins and ir<strong>on</strong> in these and many othercountries today.1.3.2.7 Vitamin D fortificati<strong>on</strong>The virtual eliminati<strong>on</strong> of childhood rickets in the industrialized countries hasbeen largely attributed to the additi<strong>on</strong> of vitamin D to milk, a practice that commencedin the 1930s in Canada and the United States. However, there are somesigns that rickets is re-emerging as a public health problem in these countries(55). In a recent study of African American women, a low intake of vitamin Dfortified milk was found to be a significant predictor of a high prevalence ofvitamin D deficiency (56). Vitamin D fortificati<strong>on</strong> of milk also reduces the riskof osteoporosis in the elderly, especially in higher latitude regi<strong>on</strong>s where levelsof incident ultraviolet light are lower during the winter m<strong>on</strong>ths (57,58).1.4 Advantages and limitati<strong>on</strong>s of <strong>food</strong> fortificati<strong>on</strong> as astrategy to combat MNMBeing a <strong>food</strong>-based approach, <strong>food</strong> fortificati<strong>on</strong> offers a number of advantagesover other interventi<strong>on</strong>s aimed at preventing and c<strong>on</strong>trolling MNM. Theseinclude:• If c<strong>on</strong>sumed <strong>on</strong> a regular and frequent basis, fortified <strong>food</strong>s will maintainbody stores of nutrients more efficiently and more effectively than will intermittentsupplements. Fortified <strong>food</strong>s are also better at lowering the risk of themultiple deficiencies that can result from seas<strong>on</strong>al deficits in the <strong>food</strong> supplyor a poor quality diet. This is an important advantage to growing childrenwho need a sustained supply of micr<strong>on</strong>utrients for growth and development,and to women of fertile age who need to enter periods of pregnancy and20
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEMlactati<strong>on</strong> <strong>with</strong> adequate nutrient stores. Fortificati<strong>on</strong> can be an excellent wayof increasing the c<strong>on</strong>tent of vitamins in breast milk and thus reducing theneed for supplementati<strong>on</strong> in postpartum women and infants.• Fortificati<strong>on</strong> generally aims to supply micr<strong>on</strong>utrients in amounts that approximateto those provided by a good, well-balanced diet. C<strong>on</strong>sequently,fortified staple <strong>food</strong>s will c<strong>on</strong>tain “natural” or near natural levels of micr<strong>on</strong>utrients,which may not necessarily be the case <strong>with</strong> supplements.• Fortificati<strong>on</strong> of widely distributed and widely c<strong>on</strong>sumed <strong>food</strong>s has the potentialto improve the nutriti<strong>on</strong>al status of a large proporti<strong>on</strong> of the populati<strong>on</strong>,both poor and wealthy.• Fortificati<strong>on</strong> requires neither changes in existing <strong>food</strong> patterns – which arenotoriously difficult to achieve, especially in the short-term – nor individualcompliance.• In most settings, the delivery system for fortified <strong>food</strong>s is already in place,generally through the private sector. The global tendency towards urbanizati<strong>on</strong>means that an ever increasing proporti<strong>on</strong> of the populati<strong>on</strong>, includingthat in developing countries is c<strong>on</strong>suming industry-processed, rather thanlocally-produced, <strong>food</strong>s. This affords many countries the opportunity todevelop effective strategies to combat MNM based <strong>on</strong> the fortificati<strong>on</strong> ofcentrally-processed dietary staples that <strong>on</strong>ce would have reached <strong>on</strong>ly a verysmall proporti<strong>on</strong> of the populati<strong>on</strong>.• Multiple micr<strong>on</strong>utrient deficiencies often coexist in a populati<strong>on</strong> that has apoor diet. It follows that multiple micr<strong>on</strong>utrient fortificati<strong>on</strong> is frequentlydesirable. In most cases, it is feasible to fortify <strong>food</strong>s <strong>with</strong> several micr<strong>on</strong>utrientssimultaneously.• It is usually possible to add <strong>on</strong>e or several micr<strong>on</strong>utrients <strong>with</strong>out adding substantiallyto the total cost of the <strong>food</strong> product at the point of manufacture.• When properly regulated, fortificati<strong>on</strong> carries a minimal risk of chr<strong>on</strong>ictoxicity.• Fortificati<strong>on</strong> is often more cost-effective than other strategies, especially if thetechnology already exists and if an appropriate <strong>food</strong> distributi<strong>on</strong> system is inplace (59,60).Although it is generally recognized that <strong>food</strong> fortificati<strong>on</strong> can have an enormouspositive impact <strong>on</strong> public health, there are, however, some limitati<strong>on</strong>s to thisstrategy for MNM c<strong>on</strong>trol:• While fortified <strong>food</strong>s c<strong>on</strong>tain increased amounts of selected micr<strong>on</strong>utrients,they are not a substitute for a good quality diet that supplies adequate21
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSamounts of energy, protein, essential fats and other <strong>food</strong> c<strong>on</strong>stituents requiredfor optimal health.• A specific fortified <strong>food</strong>stuff might not be c<strong>on</strong>sumed by all members of atarget populati<strong>on</strong>. C<strong>on</strong>versely, every<strong>on</strong>e in the populati<strong>on</strong> is exposed toincreased levels of micr<strong>on</strong>utrients in <strong>food</strong>, irrespective of whether or not theywill benefit from fortificati<strong>on</strong>.• Infants and young children, who c<strong>on</strong>sume relatively small amounts of <strong>food</strong>,are less likely to be able to obtain their recommended intakes of all micr<strong>on</strong>utrientsfrom universally fortified staples or c<strong>on</strong>diments al<strong>on</strong>e; fortified complementary<strong>food</strong>s may be appropriate for these age groups. It is also likelythat in many locati<strong>on</strong>s fortified <strong>food</strong>s will not supply adequate amountsof some micr<strong>on</strong>utrients, such as ir<strong>on</strong> for pregnant women, in which casesupplements will still be needed to satisfy the requirements of selectedpopulati<strong>on</strong> groups.• Fortified <strong>food</strong>s often fail to reach the poorest segments of the general populati<strong>on</strong>who are at the greatest risk of micr<strong>on</strong>utrient deficiency. This is becausesuch groups often have restricted access to fortified <strong>food</strong>s due to low purchasingpower and an underdeveloped distributi<strong>on</strong> channel. Many undernourishedpopulati<strong>on</strong> groups often live <strong>on</strong> the margins of the marketec<strong>on</strong>omy, relying <strong>on</strong> own-grown or locally produced <strong>food</strong>. Availability, accessand c<strong>on</strong>sumpti<strong>on</strong> of adequate quantities and a variety of micr<strong>on</strong>utrient-rich<strong>food</strong>s, such as animal <strong>food</strong>s and fruits and vegetables, is limited. Access tothe <strong>food</strong> distributi<strong>on</strong> system is similarly restricted and these populati<strong>on</strong>groups will purchase <strong>on</strong>ly small amounts of processed <strong>food</strong>s. Rice producti<strong>on</strong>,in particular, tends to be domestic or local, as does maize producti<strong>on</strong>.In populati<strong>on</strong>s who rely <strong>on</strong> these staples, it may be difficult to find an appropriate<strong>food</strong> to fortify. Fortificati<strong>on</strong> of sugar, sauces, seas<strong>on</strong>ings and otherc<strong>on</strong>diments may provide a soluti<strong>on</strong> to this problem in some countries, if suchproducts are c<strong>on</strong>sumed in sufficient amounts by target groups.• Very low-income populati<strong>on</strong> groups are known to have coexisting multiplemicr<strong>on</strong>utrient deficiencies, as a result of inadequate intakes of the traditi<strong>on</strong>aldiet. Although multiple micr<strong>on</strong>utrient fortificati<strong>on</strong> is technically possible, thereality is that the poor will be unable to obtain recommended intakes of allmicr<strong>on</strong>utrients from fortified <strong>food</strong>s al<strong>on</strong>e.• Technological issues relating to <strong>food</strong> fortificati<strong>on</strong> have yet to be fully resolved,especially <strong>with</strong> regard to appropriate levels of nutrients, stability of fortificants,nutrient interacti<strong>on</strong>s, physical properties, as well as acceptability byc<strong>on</strong>sumers including cooking properties and taste (see Part III).22
1. MICRONUTRIENT MALNUTRITION: A PUBLIC HEALTH PROBLEM• The nature of the <strong>food</strong> vehicle, and/or the fortificant, may limit the amountof fortificant that can be successfully added. For example, some ir<strong>on</strong> fortificantschange the colour and flavour of many <strong>food</strong>s to which they are added,and can cause the destructi<strong>on</strong> of fortificant vitamin A and iodine. Ways ofsolving some of these problems (e.g. microencapsulati<strong>on</strong> of fortificants <strong>with</strong>protective coatings) have been developed, but some difficulties remain (seePart III).• While it is generally possible to add a mixture of vitamins and minerals to relativelyinert and dry <strong>food</strong>s, such as cereals, interacti<strong>on</strong>s can occur betweenfortificant nutrients that adversely affect the organoleptic qualities of the <strong>food</strong>or the stability of the nutrients. Knowledge is lacking about the quantitativeimpact of interacti<strong>on</strong>s am<strong>on</strong>g nutrients that are added as a mixture <strong>on</strong> theabsorpti<strong>on</strong> of the individual nutrients. This complicates the estimati<strong>on</strong> of howmuch of each nutrient should be added. For example, the presence of largeamounts of calcium can inhibit the absorpti<strong>on</strong> of ir<strong>on</strong> from a fortified <strong>food</strong>;the presence of vitamin C has the opposite effect and increases ir<strong>on</strong>absorpti<strong>on</strong>.• Although often more cost-effective than other strategies, there are neverthelesssignificant costs associated <strong>with</strong> the <strong>food</strong> fortificati<strong>on</strong> process, whichmight limit the implementati<strong>on</strong> and effectiveness of <strong>food</strong> fortificati<strong>on</strong> programmes.These typically include start-up costs, the expense of c<strong>on</strong>ductingtrials for micr<strong>on</strong>utrient levels, physical qualities and taste, a realistic analysisof the purchasing power of the expected beneficiaries, the recurrent costsinvolved in creating and maintaining the demand for these products, as wellas the cost of an effective nati<strong>on</strong>al surveillance system to ensure that fortificati<strong>on</strong>is both effective and safe (see Chapter 9).To ensure their success and sustainability, especially in resource-poor countries,<strong>food</strong> fortificati<strong>on</strong> programmes should be implemented in c<strong>on</strong>cert <strong>with</strong> povertyreducti<strong>on</strong> programmes and other agricultural, health, educati<strong>on</strong> and social interventi<strong>on</strong>programmes that promote the c<strong>on</strong>sumpti<strong>on</strong> and utilizati<strong>on</strong> of adequatequantities of good quality nutritious <strong>food</strong>s am<strong>on</strong>g the nutriti<strong>on</strong>ally vulnerable.Food fortificati<strong>on</strong> should thus be viewed as a complementary strategy forimproving micr<strong>on</strong>utrient status.23
CHAPTER 2Food fortificati<strong>on</strong>: basic principlesFood fortificati<strong>on</strong> is usually regarded as the deliberate additi<strong>on</strong> of <strong>on</strong>e or moremicr<strong>on</strong>utrients to particular <strong>food</strong>s, so as to increase the intake of these micr<strong>on</strong>utrient(s)in order to correct or prevent a dem<strong>on</strong>strated deficiency and providea health benefit. The extent to which a nati<strong>on</strong>al or regi<strong>on</strong>al <strong>food</strong> supply is fortifiedvaries c<strong>on</strong>siderably. The c<strong>on</strong>centrati<strong>on</strong> of just <strong>on</strong>e micr<strong>on</strong>utrient might beincreased in a single <strong>food</strong>stuff (e.g. the iodizati<strong>on</strong> of salt), or, at the other endof the scale, there might be a whole range of <strong>food</strong>–micr<strong>on</strong>utrient combinati<strong>on</strong>s.The public health impact of <strong>food</strong> fortificati<strong>on</strong> depends <strong>on</strong> a number of parameters,but predominantly the level of fortificati<strong>on</strong>, the bioavailability of the fortificants,and the amount of fortified <strong>food</strong> c<strong>on</strong>sumed. As a general rule, however,the more widely and regularly a fortified <strong>food</strong> is c<strong>on</strong>sumed, the greater the proporti<strong>on</strong>of the populati<strong>on</strong> likely to benefit from <strong>food</strong> fortificati<strong>on</strong>.2.1 Terminology2.1.1 Food fortificati<strong>on</strong>For the purpose of these guidelines, <strong>food</strong> fortificati<strong>on</strong> is defined as the practiceof deliberately increasing the c<strong>on</strong>tent of essential 1 micr<strong>on</strong>utrients – that is to say,vitamins and minerals (including trace elements) – in a <strong>food</strong> so as to improvethe nutriti<strong>on</strong>al quality of the <strong>food</strong> supply and to provide a public health benefit<strong>with</strong> minimal risk to health. The public health benefits of fortificati<strong>on</strong> may eitherbe dem<strong>on</strong>strable, or indicated as potential or plausible by generally accepted scientificresearch, and include:• Preventi<strong>on</strong> or minimizati<strong>on</strong> of the risk of occurrence of micr<strong>on</strong>utrient deficiencyin a populati<strong>on</strong> or specific populati<strong>on</strong> groups.• C<strong>on</strong>tributi<strong>on</strong> to the correcti<strong>on</strong> of a dem<strong>on</strong>strated micr<strong>on</strong>utrient deficiency ina populati<strong>on</strong> or specific populati<strong>on</strong> groups.1The word “essential” means any substance that is normally c<strong>on</strong>sumed as a c<strong>on</strong>stituent of <strong>food</strong>which is needed for growth and development and the maintenance of healthy life and whichcannot be synthesized in adequate amounts by the body (61).24
2. FOOD FORTIFICATION: BASIC PRINCIPLES• A potential for an improvement in nutriti<strong>on</strong>al status and dietary intakes thatmay be, or may become, suboptimal as a result of changes in dietaryhabits/lifestyles.• Plausible beneficial effects of micr<strong>on</strong>utrients c<strong>on</strong>sistent <strong>with</strong> maintaining orimproving health (e.g. there is some evidence to suggest that a diet rich inselected anitoxidants might help to prevent cancer and other diseases).The Codex General Principles for the Additi<strong>on</strong> of Essential Nutrients to Foods (61)defines “fortificati<strong>on</strong>”, or syn<strong>on</strong>ymously “enrichment”, as “the additi<strong>on</strong> of <strong>on</strong>eor more essential nutrients to a <strong>food</strong> whether or not it is normally c<strong>on</strong>tained inthe <strong>food</strong>, for the purpose of preventing or correcting a dem<strong>on</strong>strated deficiencyof <strong>on</strong>e or more nutrients in the populati<strong>on</strong> or specific populati<strong>on</strong> groups”.The Codex General Principles go <strong>on</strong> to state that the first-menti<strong>on</strong>ed c<strong>on</strong>diti<strong>on</strong>for the fulfilment of any fortificati<strong>on</strong> programme “should be a dem<strong>on</strong>stratedneed for increasing the intake of an essential nutrient in <strong>on</strong>e or more populati<strong>on</strong>groups. This may be in the form of actual clinical or subclinical evidenceof deficiency, estimates indicating low levels of intake of nutrients or possibledeficiencies likely to develop because of changes taking place in <strong>food</strong>habits” (61).The broad definiti<strong>on</strong> of fortificati<strong>on</strong> used in these guidelines extends the interpretati<strong>on</strong>of public health need prescribed by the Codex General Principles forthe Additi<strong>on</strong> of Essential Nutrients to Foods (61) in that it also incorporates plausiblepublic health benefits that may be derived from increased micr<strong>on</strong>utrientintakes (as opposed to merely dem<strong>on</strong>strable benefits), based <strong>on</strong> new and evolvingscientific knowledge. The broader definiti<strong>on</strong> thus encompasses the growingrange of different types of <strong>food</strong> fortificati<strong>on</strong> initiatives that have been implementedin recent years in resp<strong>on</strong>se to an increasingly diverse set of public healthcircumstances.Clearly, the public health significance of the potential benefits of <strong>food</strong>fortificati<strong>on</strong> is primarily a functi<strong>on</strong> of the extent of the public health problem.Generally speaking, therefore, when deciding to implement a fortificati<strong>on</strong>programme, priority should be given to c<strong>on</strong>trolling those nutrient deficienciesthat are most comm<strong>on</strong> in the populati<strong>on</strong> and that have the greatest adverse effect<strong>on</strong> health and functi<strong>on</strong>. In Part II of these guidelines, appropriate criteria thatcan be applied to the determinati<strong>on</strong> of the significance of the public healthproblem are described; these criteria are largely expressed in terms of the prevalenceand severity of MNM. Ideally this should be determined at the countryor regi<strong>on</strong>al level.2.1.2 Related codex terminologyThe following definiti<strong>on</strong>s are used in these guidelines as follows:25
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS• Restorati<strong>on</strong> is the additi<strong>on</strong> of essential nutrients to <strong>food</strong>s to restore amountsoriginally present in the natural product that are unavoidably lost during processing(e.g. milling), storage or handling.• Nutriti<strong>on</strong>al equivalence is achieved when an essential nutrient is added to aproduct that is designed to resemble a comm<strong>on</strong> <strong>food</strong> in appearance, texture,flavour and odour in amounts such that the substitute product has a similarnutritive value, in terms of the amount and bioavailability of the added essentialnutrient. An example is the additi<strong>on</strong> of vitamin A to margarine sold as abutter substitute, in an amount equal to butter’s natural c<strong>on</strong>tent.• Appropriate nutrient compositi<strong>on</strong> of a special purpose <strong>food</strong> describes the additi<strong>on</strong>of an essential nutrient to a <strong>food</strong> that is designed to perform a specific functi<strong>on</strong>(such as meal replacement or a complementary <strong>food</strong> for young children),or that is processed or formulated to satisfy particular dietary requirements,in amounts that ensure that the nutrient c<strong>on</strong>tent of the <strong>food</strong> is adequate andappropriate for its purpose.Whereas restorati<strong>on</strong> and nutriti<strong>on</strong>al equivalence are strategies aimed at correcting<strong>food</strong> supply changes that could otherwise adversely affect public health, theterm “fortificati<strong>on</strong>” tends to be reserved for essential nutrient additi<strong>on</strong>s thataddress specific public health needs. Nevertheless, all the Codex categories ofnutrient additi<strong>on</strong>s adopt, albeit to a varying degree, the general aim of providinga public health benefit.2.2 Types of fortificati<strong>on</strong>Food fortificati<strong>on</strong> can take several forms. It is possible to fortify <strong>food</strong>s that arewidely c<strong>on</strong>sumed by the general populati<strong>on</strong> (mass fortificati<strong>on</strong> 1 ), to fortify <strong>food</strong>sdesigned for specific populati<strong>on</strong> subgroups, such as complementary <strong>food</strong>s foryoung children or rati<strong>on</strong>s for displaced populati<strong>on</strong>s (targeted fortificati<strong>on</strong>)and/or to allow <strong>food</strong> manufacturers to voluntarily fortify <strong>food</strong>s available in themarket place (market-driven fortificati<strong>on</strong> 2 ).Generally speaking, mass fortificati<strong>on</strong> is nearly always mandatory, targetedfortificati<strong>on</strong> can be either mandatory or voluntary depending <strong>on</strong> the publichealth significance of the problem it is seeking to address, and market-drivenfortificati<strong>on</strong> is always voluntary, but governed by regulatory limits (Figure 2.1).The choice between mandatory or voluntary <strong>food</strong> fortificati<strong>on</strong> usually depends<strong>on</strong> nati<strong>on</strong>al circumstances. For example, in countries where a large proporti<strong>on</strong>of maize flour is produced by small mills, enforcement of mandatory fortifica-1Mass fortificati<strong>on</strong> is sometimes called “universal fortificati<strong>on</strong>”.2Market-driven fortificati<strong>on</strong> is sometimes called “industry-driven fortificati<strong>on</strong>”, “open-market” or“free-market” fortificati<strong>on</strong>.26
2. FOOD FORTIFICATION: BASIC PRINCIPLESFIGURE 2.1The interrelati<strong>on</strong>ships between the levels of coverage and compliance and thedifferent types of <strong>food</strong> fortificati<strong>on</strong>General populati<strong>on</strong>VoluntaryMarketdrivenfortificati<strong>on</strong>Massfortificati<strong>on</strong>MandatoryTargetfortificati<strong>on</strong>CoverageComplianceSpecific groupsti<strong>on</strong> might be impractical. Under such circumstances, <strong>on</strong>e opti<strong>on</strong> would be, iffeasible, to allow small mills to fortify their product <strong>on</strong> a voluntary basis but followingspecified regulati<strong>on</strong>s.2.2.1 Mass fortificati<strong>on</strong>As indicated above, mass fortificati<strong>on</strong> is the term used to describe the additi<strong>on</strong>of <strong>on</strong>e or more micr<strong>on</strong>utrients to <strong>food</strong>s comm<strong>on</strong>ly c<strong>on</strong>sumed by the generalpublic, such as cereals, c<strong>on</strong>diments and milk. It is usually instigated, mandatedand regulated by the government sector.Mass fortificati<strong>on</strong> is generally the best opti<strong>on</strong> when the majority of the populati<strong>on</strong>has an unacceptable risk, in terms of public health, of being or becomingdeficient in specific micr<strong>on</strong>utrients. In some situati<strong>on</strong>s, deficiency may bedem<strong>on</strong>strable, as evidenced by unacceptably low intakes and/or biochemicalsigns of deficiency. In others, the populati<strong>on</strong> may not actually be deficientaccording to usual biochemical or dietary criteria, but are likely to benefit fromfortificati<strong>on</strong>. The mandatory additi<strong>on</strong> of folic acid to wheat flour <strong>with</strong> a view tolowering the risk of birth defects, a practice which has been introduced inCanada and the United States, and also in many Latin American countries, is<strong>on</strong>e example of the latter scenario.2.2.2 Targeted fortificati<strong>on</strong>In targeted <strong>food</strong> fortificati<strong>on</strong> programmes, <strong>food</strong>s aimed at specific subgroups ofthe populati<strong>on</strong> are fortified, thereby increasing the intake of that particular group27
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 2.1Targeted <strong>food</strong> fortificati<strong>on</strong> programmesCountry Food Target populati<strong>on</strong>Guatemala IncaparinaInd<strong>on</strong>esia Complementary <strong>food</strong>s InfantsMexicoProgresaPeru Ali Alimentu SchoolchildrenSouth Africa Biscuits Schoolchildrenrather than that of the populati<strong>on</strong> as a whole. Examples include complementary<strong>food</strong>s for infants and young children, <strong>food</strong>s developed for school feeding programmes,special biscuits for children and pregnant women, and rati<strong>on</strong>s(blended <strong>food</strong>s) for emergency feeding and displaced pers<strong>on</strong>s (Table 2.1). Insome cases, such <strong>food</strong>s may be required to provide a substantial proporti<strong>on</strong> ofdaily micr<strong>on</strong>utrient requirements of the target group.The majority of blended <strong>food</strong>s for feeding refugees and displaced pers<strong>on</strong>s aremanaged by the World Food Programme (WFP) and guidelines covering theirfortificati<strong>on</strong> (including wheat soy blends and corn soy blends) are already available(62). Although blended <strong>food</strong>s usually supply all or nearly all of the energyand protein intake of refugees and displaced individuals, especially in the earlierstages of dislocati<strong>on</strong>, for historical reas<strong>on</strong>s such <strong>food</strong>s may not always provideadequate amounts of all micr<strong>on</strong>utrients. Therefore, other sources of micr<strong>on</strong>utrientsmay need to be provided. In particular, it may be necessary to add iodizedsalt to <strong>food</strong>s, provide ir<strong>on</strong> supplements to pregnant women or supply high-dosevitamin A supplements to young children and postpartum women. Wheneverpossible, fresh fruits and vegetables should be added to the diets of displacedpers<strong>on</strong>s relying <strong>on</strong> blended <strong>food</strong>s (see Chapter 4: secti<strong>on</strong> 4.5). Fortified <strong>food</strong>sfor displaced pers<strong>on</strong>s are often targeted at children and pregnant or lactatingwomen.2.2.3 Market-driven fortificati<strong>on</strong>The term “market-driven fortificati<strong>on</strong>” is applied to situati<strong>on</strong>s whereby a <strong>food</strong>manufacturer takes a business-oriented initiative to add specific amounts of <strong>on</strong>eor more micr<strong>on</strong>utrients to processed <strong>food</strong>s. Although voluntary, this type of <strong>food</strong>fortificati<strong>on</strong> usually takes place <strong>with</strong>in government-set regulatory limits (seeChapter 11: Nati<strong>on</strong>al <strong>food</strong> law).Market-driven fortificati<strong>on</strong> can play a positive role in public health by c<strong>on</strong>tributingto meeting nutrient requirements and thereby reducing the risk ofmicr<strong>on</strong>utrient deficiency. In the European Uni<strong>on</strong>, fortified processed <strong>food</strong>s havebeen shown to be a substantial source of micr<strong>on</strong>utrients such as ir<strong>on</strong>, and vita-28
2. FOOD FORTIFICATION: BASIC PRINCIPLESmins A and D (63,64). Market-driven fortificati<strong>on</strong> can also improve the supplyof micr<strong>on</strong>utrients that are otherwise difficult to add in sufficient amountsthrough the mass fortificati<strong>on</strong> of staple <strong>food</strong>s and c<strong>on</strong>diments because of safety,technological or cost c<strong>on</strong>straints. Examples include certain minerals (e.g. ir<strong>on</strong>,calcium) and sometimes selected vitamins (e.g. vitamin C, vitamin B 2 ).Market-driven fortificati<strong>on</strong> is more widespread in industrialized countries,whereas in most developing countries the public health impact of market-driven<strong>food</strong> interventi<strong>on</strong>s is still rather limited. However, their importance is likely tobe greater in the future, because of increasing urbanizati<strong>on</strong> and wider availabilityof such <strong>food</strong>s.The predicted increase in the availability of fortified processed <strong>food</strong>s in developingcountries has given rise to a number of c<strong>on</strong>cerns. Firstly, these fortified<strong>food</strong>s – especially those that are attractive to c<strong>on</strong>sumers – could divert c<strong>on</strong>sumersfrom their usual dietary pattern and result in, for example, an increasedc<strong>on</strong>sumpti<strong>on</strong> of sugar, or a lower c<strong>on</strong>sumpti<strong>on</strong> of fibre. Sec<strong>on</strong>dly, because inmost developing countries <strong>food</strong>s fortified through market-driven fortificati<strong>on</strong>currently receive scant regulatory attenti<strong>on</strong> even though such <strong>food</strong>s are intendedfor wide-scale c<strong>on</strong>sumpti<strong>on</strong> (see secti<strong>on</strong> 2.3), there is a potential risk that unnecessarilyhigh levels of micr<strong>on</strong>utrients may be delivered to children if the sameserving size of the fortified <strong>food</strong> (such as breakfast cereals, beverages and nutriti<strong>on</strong>bars) is intended for all members of a household. Regulati<strong>on</strong> is thus necessaryto ensure that the c<strong>on</strong>sumpti<strong>on</strong> of these <strong>food</strong>s will not result in anexcessive intake of micr<strong>on</strong>utrients. Furthermore, manufacturers of processedfortified <strong>food</strong>s should be encouraged to follow the same quality c<strong>on</strong>trol andassurance procedures as those that are prescribed for mandatory mass-fortifiedproducts (see Chapter 8: M<strong>on</strong>itoring and evaluati<strong>on</strong>).2.2.4 Other types of fortificati<strong>on</strong>2.2.4.1 Household and community fortificati<strong>on</strong>Efforts are under way in a number of countries to develop and test practicalways of adding micr<strong>on</strong>utrients to <strong>food</strong>s at the household level, in particular, tocomplementary <strong>food</strong>s for young children. In effect, this approach is a combinati<strong>on</strong>of supplementati<strong>on</strong> and fortificati<strong>on</strong>, and has been referred to by someas “complementary <strong>food</strong> supplementati<strong>on</strong>” (65).The efficacy and effectiveness of several different types of products, includingsoluble or crushable tablets, micr<strong>on</strong>utrient-based powder (“sprinkles”) andmicr<strong>on</strong>utrient-rich spreads are currently being evaluated (Table 2.2). Crushabletablets, and especially micr<strong>on</strong>utrient-based powder, are relatively expensive waysof increasing micr<strong>on</strong>utrient intakes, certainly more costly than mass fortificati<strong>on</strong>,but may be especially useful for improving local <strong>food</strong>s fed to infants andyoung children, or where universal fortificati<strong>on</strong> is not possible (66). The29
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 2.2Foods for fortificati<strong>on</strong> at the household levelProductMicr<strong>on</strong>utrient powderwhich can besprinkled <strong>on</strong>to <strong>food</strong>Soluble micr<strong>on</strong>utrienttablets which can bedissolved in waterand fed as a drinkCrushable micr<strong>on</strong>utrienttablets for adding to<strong>food</strong>sFat-based spreadfortified <strong>with</strong>micr<strong>on</strong>utrientComments■ C<strong>on</strong>tain several micr<strong>on</strong>utrients, including ir<strong>on</strong>, encapsulated tominimize adverse interacti<strong>on</strong>s between micr<strong>on</strong>utrients andsensory changes to the <strong>food</strong> to which they are added;available in sachets■ Suitable for young children;■ Tested by WHO■ For infants and young children■ Tested by UNICEF■ Popular <strong>with</strong> children■ Can be produced locally as the technology required is easyto implementSources: references (66,67).micr<strong>on</strong>utrient-dense fortified spreads have been found to be very popular <strong>with</strong>children (67).Fortificati<strong>on</strong> of <strong>food</strong>s at the community level is also still at the experimentalstage. One such approach involves the additi<strong>on</strong> of a commercial micr<strong>on</strong>utrientpremix, available in sachets, to small batches of flour during the milling process(68). Although feasible in theory, major challenges to local-scale fortificati<strong>on</strong>programmes include the initial cost of the mixing equipment, the price of thepremix (which would need to be imported in most cases), achieving and maintainingan adequate standard of quality c<strong>on</strong>trol (e.g. in uniformity of mixing),and sustaining m<strong>on</strong>itoring and distributi<strong>on</strong> systems.2.2.4.2 Biofortificati<strong>on</strong> of staple <strong>food</strong>sThe biofortificati<strong>on</strong> of staple <strong>food</strong>s, i.e. the breeding and genetic modificati<strong>on</strong>of plants so as to improve their nutrient c<strong>on</strong>tent and/or absorpti<strong>on</strong> is anothernovel approach that is currently being c<strong>on</strong>sidered. The potential for plant breedingto increase the micr<strong>on</strong>utrient c<strong>on</strong>tent of various cereals, legumes and tuberscertainly exists; for instance, it is possible to select certain cereals (such as rice)and legumes for their high ir<strong>on</strong> c<strong>on</strong>tent, various varieties of carrots and sweetpotatoes for their favourable β-carotene levels, and maizes for their low phytatec<strong>on</strong>tent (which improves the absorpti<strong>on</strong> of ir<strong>on</strong> and zinc) (69–71). However,much more work still needs to be d<strong>on</strong>e before the efficacy and effectiveness ofthese <strong>food</strong>s are proven, and current c<strong>on</strong>cerns about their safety, cost and impact<strong>on</strong> the envir<strong>on</strong>ment are alleviated (72).30
2. FOOD FORTIFICATION: BASIC PRINCIPLES2.3 Legal c<strong>on</strong>siderati<strong>on</strong>s: mandatory versusvoluntary fortificati<strong>on</strong>The huge diversity in nati<strong>on</strong>al circumstances and public health goals worldwidehas resulted in the development of many different approaches to the regulati<strong>on</strong>of <strong>food</strong> fortificati<strong>on</strong>. In most industrialized countries, <strong>food</strong> fortificati<strong>on</strong> parametersare established by law or through cooperative arrangements. Elsewhere,and representing the other end of the spectrum, fortified <strong>food</strong>s are produced<strong>with</strong>out any form of governmental guidance or c<strong>on</strong>trol at all. Since it is the roleof government to protect public health, it is generally recommended that allforms of <strong>food</strong> fortificati<strong>on</strong> be appropriately regulated in order to ensure thesafety of all c<strong>on</strong>sumers and the maximum benefit to target groups.Within the legal c<strong>on</strong>text, fortificati<strong>on</strong> can be categorized as either mandatoryor voluntary. These terms refer to the level of obligati<strong>on</strong> required of <strong>food</strong> producersto comply <strong>with</strong> government intenti<strong>on</strong>s expressed in law.The fundamental distincti<strong>on</strong> between mandatory and voluntary regulati<strong>on</strong> asit applies to <strong>food</strong> fortificati<strong>on</strong> is the level of certainty over time that a particularcategory of <strong>food</strong> will c<strong>on</strong>tain a pre-determined amount of a micr<strong>on</strong>utrient. Byproviding a higher level of certainty, mandatory fortificati<strong>on</strong> is more likely todeliver a sustained source of fortified <strong>food</strong> for c<strong>on</strong>sumpti<strong>on</strong> by the relevant populati<strong>on</strong>group, and, in turn, a public health benefit.2.3.1 Mandatory fortificati<strong>on</strong>2.3.1.1 Key characteristicsMandatory fortificati<strong>on</strong> occurs when governments legally oblige <strong>food</strong> producersto fortify particular <strong>food</strong>s or categories of <strong>food</strong>s <strong>with</strong> specified micr<strong>on</strong>utrients.Mandatory fortificati<strong>on</strong>, especially when supported by a properlyresourced enforcement and informati<strong>on</strong> disseminati<strong>on</strong> system, delivers a highlevel of certainty that the selected <strong>food</strong>(s) will be appropriately fortified and inc<strong>on</strong>stant supply.In deciding the precise form of mandatory fortificati<strong>on</strong> regulati<strong>on</strong>, governmentsare resp<strong>on</strong>sible for ensuring that the combinati<strong>on</strong> of the <strong>food</strong> vehicleand the fortificants will be both efficacious and effective for the target group,yet safe for target and n<strong>on</strong>-target groups alike. Food vehicles range from basiccommodities, such as various types of flour, sugar and salt which are available<strong>on</strong> the retail market for use by c<strong>on</strong>sumers as well as ingredients of processed<strong>food</strong>s, to processed <strong>food</strong>s that are fortified at the point of manufacture.Given their widespread and regular c<strong>on</strong>sumpti<strong>on</strong>, basic commodities aremore suited to mass fortificati<strong>on</strong> (i.e. intended to reach the whole populati<strong>on</strong>),whereas certain processed formulated <strong>food</strong>s are usually the better vehiclefor targeted fortificati<strong>on</strong> initiatives (i.e. those aimed at specific populati<strong>on</strong>groups).31
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSGlobally, mandatory regulati<strong>on</strong>s are most often applied to the fortificati<strong>on</strong> of<strong>food</strong> <strong>with</strong> micr<strong>on</strong>utrients such as iodine, ir<strong>on</strong>, vitamin A, and increasingly folicacid. Of these, the iodizati<strong>on</strong> of salt is probably the most widely adopted formof mandatory mass fortificati<strong>on</strong>. In the Philippines, for example, the legal standardfor iodized salt, which is appended to the Philippine Act Promoting SaltIodizati<strong>on</strong> Nati<strong>on</strong>wide, requires a minimum level of iodine fortificati<strong>on</strong> of all<strong>food</strong>-grade salt destined for human c<strong>on</strong>sumpti<strong>on</strong> (6). This form of mandatoryregulati<strong>on</strong> is used in many other countries. Other examples of mandatory massfortificati<strong>on</strong> include the additi<strong>on</strong> of vitamin A to sugar and margarine, and thefortificati<strong>on</strong> of flour <strong>with</strong> ir<strong>on</strong> (usually together <strong>with</strong> restorati<strong>on</strong> of vitamins B 1 ,B 2 and niacin), and, more recently, <strong>with</strong> folic acid and vitamin B 12 .The types of <strong>food</strong> vehicles that are subjected to mandatory fortificati<strong>on</strong>are usually characterized by either a physical or an intrinsic attribute, or a specificpurpose. A requisite flour, for example, could be described as eitherwhite or wholemeal and/or milled from a particular grain, or destined forbread making. Alternatively, the prescribed fortificati<strong>on</strong> requirements mayapply <strong>on</strong>ly to a <strong>food</strong> that is identified and labelled in a certain way. In the UnitedStates, for instance, <strong>on</strong>ly those flours and other grain products identifiedand labelled as “enriched” are required by law to c<strong>on</strong>tain added folic acid (andsome other essential micr<strong>on</strong>utrients). Similarly, Australia and New Zealandmandate the additi<strong>on</strong> of iodine <strong>on</strong>ly to salt identified and labelled as “iodizedsalt”. Although the potential public health impact is more variable, mass fortificati<strong>on</strong>can be achieved under these c<strong>on</strong>diti<strong>on</strong>s, particularly if the labelled fortified<strong>food</strong>s c<strong>on</strong>stitute a major and stable share of the market for that <strong>food</strong> classas a whole.2.3.1.2 Mandatory fortificati<strong>on</strong> in relati<strong>on</strong> to public healthGovernments tend to institute mandatory fortificati<strong>on</strong> in situati<strong>on</strong>s where a proporti<strong>on</strong>of the general populati<strong>on</strong> – either the majority (mass fortificati<strong>on</strong>) or anidentified populati<strong>on</strong> group (target fortificati<strong>on</strong>) – has a significant public healthneed, or is at risk of being, or becoming, deficient in a specific micr<strong>on</strong>utrient(s),and where such needs or risks can be ameliorated or minimized by a sustainedsupply and regular c<strong>on</strong>sumpti<strong>on</strong> of particular fortified <strong>food</strong>(s) c<strong>on</strong>taining thosemicr<strong>on</strong>utrients.Mandatory fortificati<strong>on</strong> is usually prompted by evidence that a given populati<strong>on</strong>is deficient or inadequately nourished, such as clinical or biochemical signsof deficiency and/or unacceptably low levels of micr<strong>on</strong>utrient intake. In somecircumstances, a dem<strong>on</strong>strated public health benefit of an increased c<strong>on</strong>sumpti<strong>on</strong>of a given micr<strong>on</strong>utrient might be c<strong>on</strong>sidered sufficient grounds to warrantmandatory fortificati<strong>on</strong> even if the populati<strong>on</strong> is not c<strong>on</strong>sidered to be seriouslyat risk according to c<strong>on</strong>venti<strong>on</strong>al biochemical or dietary intake criteria. The32
2. FOOD FORTIFICATION: BASIC PRINCIPLESmandatory additi<strong>on</strong> of folic acid to flour to reduce the risk of birth defects is acase in point.2.3.2 Voluntary fortificati<strong>on</strong>2.3.2.1 Key characteristicsFortificati<strong>on</strong> is described as voluntary when a <strong>food</strong> manufacturer freely choosesto fortify particular <strong>food</strong>s in resp<strong>on</strong>se to permissi<strong>on</strong> given in <strong>food</strong> law, or underspecial circumstances, is encouraged by government to do so.The impetus for voluntary fortificati<strong>on</strong> usually stems from industry and c<strong>on</strong>sumersseeking to obtain possible health benefits through an increase inmicr<strong>on</strong>utrient intakes. Occasi<strong>on</strong>ally, however, government provides the drivingforce. Given this diversity in the circumstances that drive voluntary fortificati<strong>on</strong>,it is not surprising that the public health impacts range from negligible to substantial.Indeed, depending <strong>on</strong> the nutriti<strong>on</strong>al quality of their basic diet, thoseindividuals who regularly c<strong>on</strong>sume fortified <strong>food</strong>s might well gain discernablebenefits.However, it is important that governments exercise an appropriate degree ofc<strong>on</strong>trol over voluntary fortificati<strong>on</strong> through <strong>food</strong> laws or other cooperativearrangements, such as industry codes of practice. The degree of c<strong>on</strong>trol shouldat least be commensurate <strong>with</strong> the inherent level of risk. Regulatory c<strong>on</strong>trols ofthis nature should also ensure the safety of fortified <strong>food</strong>s for all c<strong>on</strong>sumers, aswell as provide opportunities for industry to produce fortified <strong>food</strong>s that offerc<strong>on</strong>sumers nutriti<strong>on</strong>al and/or other health benefits. The potential benefits maybe dem<strong>on</strong>strable, or indicated as potential or plausible by generally acceptedscientific data.When instituting voluntary fortificati<strong>on</strong> arrangements, governments have aduty to ensure that c<strong>on</strong>sumers are not misled or deceived by fortificati<strong>on</strong> practicesand may also wish to be satisfied that market promoti<strong>on</strong> of fortified <strong>food</strong>sdoes not c<strong>on</strong>flict <strong>with</strong>, or compromise, any nati<strong>on</strong>al <strong>food</strong> and nutriti<strong>on</strong> policies<strong>on</strong> healthy eating. This could be achieved through regulati<strong>on</strong>s <strong>on</strong> the range of<strong>food</strong>s eligible for voluntary fortificati<strong>on</strong> and <strong>on</strong> the permitted combinati<strong>on</strong>s ofparticular micr<strong>on</strong>utrients and <strong>food</strong>s (see Chapter 11: Nati<strong>on</strong>al <strong>food</strong> law).Currently many countries permit voluntary fortificati<strong>on</strong>, but the range of<strong>food</strong>s that may be fortified varies c<strong>on</strong>siderably from country to country. SomeScandinavian countries allow <strong>on</strong>ly a narrow range of <strong>food</strong>s to be fortified,whereas the range of products that can be fortified is much greater in the UnitedStates. Similarly, the permitted fortificants range from a select few to almost allmicr<strong>on</strong>utrients that are c<strong>on</strong>sidered essential.The level of industry uptake of fortificati<strong>on</strong> practice is greatly influenced byprevailing market c<strong>on</strong>diti<strong>on</strong>s. For example, in many industrialized countries, thevast majority of processed breakfast cereals are moderately fortified <strong>with</strong> various33
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTScombinati<strong>on</strong>s of micr<strong>on</strong>utrients, sometimes differentiated according to the targetmarket; the remaining few are either highly or extensively fortified (where permitted)and/or unfortified. Other <strong>food</strong> categories, such as fruit juices or dairyproducts, tend to exhibit a greater variability in fortificati<strong>on</strong> rates, this beinginfluenced by market differentiati<strong>on</strong> and brand identity. For some permitted categoriesthere may be no industry interest in fortificati<strong>on</strong>.2.3.2.2 Voluntary fortificati<strong>on</strong> in relati<strong>on</strong> to public healthVoluntary fortificati<strong>on</strong> tends to be used when there are lower order risks to publichealth, i.e. when the risks to public health are not as serious or dem<strong>on</strong>strable soas to warrant mass fortificati<strong>on</strong>. Inadequate micr<strong>on</strong>utrient intakes that arisebecause of changes in lifestyles that tend to follow changing social and ec<strong>on</strong>omiccircumstances are more likely to be associated <strong>with</strong> lower order public healthrisks than inadequate intakes that arise because of significantly modified eatinghabits and dietary behaviour. In additi<strong>on</strong>, for certain nutrients, dietary requirementshave been reappraised in light of evolving scientific knowledge about theirphysiological role and the beneficial effects <strong>on</strong> certain physiological processesand health c<strong>on</strong>diti<strong>on</strong>s.Because of uncertainty about the level of industry uptake of fortificati<strong>on</strong><strong>with</strong>in each <strong>food</strong> product category, and the fact that regular c<strong>on</strong>sumers of agiven fortified <strong>food</strong> may vary over time and thus do not c<strong>on</strong>stitute a readily identifiablegroup, voluntary fortificati<strong>on</strong> is less likely than mandatory fortificati<strong>on</strong>to deliver a guaranteed favourable outcome in terms of increased intakes ofmicr<strong>on</strong>utrients across a target populati<strong>on</strong>. Apart from the extent to which a given<strong>food</strong> category is fortified, the public health impact of voluntary fortificati<strong>on</strong>depends <strong>on</strong> the c<strong>on</strong>tributi<strong>on</strong> of that <strong>food</strong> category to the diet of the populati<strong>on</strong>as whole, and also whether or not those individuals who would benefit most fromfortificati<strong>on</strong> regularly c<strong>on</strong>sume and have access to that <strong>food</strong> category.Despite these inherent difficulties, a c<strong>on</strong>sistent supply of appropriatelyregulated, voluntarily-fortified <strong>food</strong>s, produced under free-market c<strong>on</strong>diti<strong>on</strong>sand widely and regularly c<strong>on</strong>sumed by a given populati<strong>on</strong> group, can have abeneficial impact <strong>on</strong> public health by positively c<strong>on</strong>tributing to micr<strong>on</strong>utrientbalance and thereby reducing the risk of deficiency. For example, in theEuropean Uni<strong>on</strong> where fortificati<strong>on</strong> of margarine is voluntary, it is estimatedthat the additi<strong>on</strong> of vitamins A and D to margarine and spreadable fats c<strong>on</strong>tributesabout 20% of the reference nutrient intake for vitamin A and 30% ofthat for vitamin D (63). It has also been reported that by the 1990s fortifiedbreakfast cereals had become the principal source of ir<strong>on</strong> for young children inthe United Kingdom (64).34
2. FOOD FORTIFICATION: BASIC PRINCIPLES2.3.3 Special voluntary fortificati<strong>on</strong>Some voluntary fortificati<strong>on</strong> programmes are capable of achieving similar outcomesto mandatory fortificati<strong>on</strong>, thus avoiding the need for complex mandatorylegal requirements. A notable example is the Swiss programme of saltiodizati<strong>on</strong>. Circumstances that c<strong>on</strong>tribute to the success of voluntary fortificati<strong>on</strong>in Switzerland and elsewhere include the existence of an industry that comprises<strong>on</strong>ly a few producers or manufacturers and a str<strong>on</strong>g government interestin industry practice (e.g. <strong>on</strong>e that provides subsidies and ensures sustainable fortificati<strong>on</strong>practices). Voluntary fortificati<strong>on</strong> initiatives are also more likely tosucceed when supported by public educati<strong>on</strong> activities that increase publicawareness of the importance of c<strong>on</strong>suming the fortified <strong>food</strong> (see Chapter 10:Communicati<strong>on</strong>, social marketing and advocacy).2.3.4 Criteria governing the selecti<strong>on</strong> of mandatory or voluntary fortificati<strong>on</strong>For any given populati<strong>on</strong> group, which may be either the entire populati<strong>on</strong> ora specific subgroup(s), there are five key factors that together determine whethermandatory or voluntary fortificati<strong>on</strong> is likely to be the most appropriate opti<strong>on</strong>for the prevailing c<strong>on</strong>diti<strong>on</strong>s. In brief, they are: the significance of the publichealth need; the size and scale of the <strong>food</strong> industry sector; the level of awarenessam<strong>on</strong>g the populati<strong>on</strong> about nutriti<strong>on</strong>al needs; the political envir<strong>on</strong>ment;and <strong>food</strong> c<strong>on</strong>sumpti<strong>on</strong> patterns. These five factors are described in more detailbelow, and in each case, an indicati<strong>on</strong> given of the circumstances that favour <strong>on</strong>eor the other of the two main regulatory mechanisms.1. The significance of the public health need or risk of deficiency, as determined bythe severity of the problem and its prevalence <strong>with</strong>in a populati<strong>on</strong> group. The significanceof the public health problem is of primary importance and shouldbe determined at the country or regi<strong>on</strong>al level, ideally <strong>with</strong> reference to setcriteria that describe the severity of the public health problem. The publichealth need or risk can be assessed according to evidence of clinical orsubclinical deficiency, inadequate nutrient intake, or potential health benefit(see Part II: Evaluating the public health significance of micr<strong>on</strong>utrientmalnutriti<strong>on</strong>).■ Mandatory fortificati<strong>on</strong> is more suited to cases of serious public health needor risk, and voluntary fortificati<strong>on</strong> to cases of lower order public health needor risk, or where the potential exists for some individuals to benefit from, orto exercise, c<strong>on</strong>sumer choice.■ Under certain c<strong>on</strong>diti<strong>on</strong>s, voluntary fortificati<strong>on</strong> can achieve similar publichealth impacts as mandatory fortificati<strong>on</strong>.35
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS2. The features of the <strong>food</strong> industry sector that will resp<strong>on</strong>sible for the producti<strong>on</strong> ofthe proposed <strong>food</strong> vehicle. The aspects of the <strong>food</strong> industry sector that are especiallyrelevant in this c<strong>on</strong>text are the number, capacity and geographical distributi<strong>on</strong>of the producers, the presence of any government support orc<strong>on</strong>trol, and the prevailing commercial envir<strong>on</strong>ment.■ In developing countries in particular, mandatory fortificati<strong>on</strong> is more likely tosucceed when the industry sector in questi<strong>on</strong> is either relatively centralized(i.e c<strong>on</strong>fined to a handful of major producers) and/or well organized. If it c<strong>on</strong>sistsof numerous small, widely dispersed producers, mandatory fortificati<strong>on</strong>will be more difficult to achieve, unless these small units have some form ofcollective arrangement in place, such as an established industry associati<strong>on</strong>.It is also the better opti<strong>on</strong> in settings where governments seeking highrates of industry participati<strong>on</strong> do not have any alternative legal or administrativearrangements that could potentially be used to institute voluntarycooperative arrangements <strong>with</strong>in the industry.■ Voluntary fortificati<strong>on</strong> does not need to take account of industry arrangementsbut where there is a m<strong>on</strong>opoly or a government-sp<strong>on</strong>sored industry,the impact of voluntary arrangements can match those achieved by mandatoryfortificati<strong>on</strong>.3. The relevant populati<strong>on</strong>’s present level of knowledge about the importance of c<strong>on</strong>sumingfortified <strong>food</strong>s or their interest in c<strong>on</strong>suming fortified <strong>food</strong>s. The level ofresources available for implementing and sustaining specific nutriti<strong>on</strong> educati<strong>on</strong>programmes is also an important factor to c<strong>on</strong>sider when choosingthe most suitable regulatory envir<strong>on</strong>ment for a <strong>food</strong> fortificati<strong>on</strong> programme.■ Mandatory fortificati<strong>on</strong> is likely to be the more effective opti<strong>on</strong> when c<strong>on</strong>sumerknowledge is poor or demand for voluntarily-fortified products is low, andthere are few opportunities for community nutriti<strong>on</strong> educati<strong>on</strong>.■ Voluntary fortificati<strong>on</strong> generally relies <strong>on</strong> c<strong>on</strong>sumer interest and/or demandfor fortified <strong>food</strong>s. Although c<strong>on</strong>sumer behaviour is influenced by manyfactors, it could be engendered by commercial promoti<strong>on</strong> or specific nutriti<strong>on</strong>educati<strong>on</strong> programmes.4. The political envir<strong>on</strong>ment. In terms of the political envir<strong>on</strong>ment, the acceptablelevel of government interventi<strong>on</strong> and the value placed <strong>on</strong> informed c<strong>on</strong>sumerchoice are probably the most significant factors that affectingregulatory decisi<strong>on</strong>s.36
2. FOOD FORTIFICATION: BASIC PRINCIPLES■ In envir<strong>on</strong>ments where c<strong>on</strong>sumer choice is highly valued, both voluntary andmandatory fortificati<strong>on</strong> could be appropriate. In such settings, mandatory fortificati<strong>on</strong>tends to be limited to a subset of products <strong>with</strong>in <strong>on</strong>e or more proposed<strong>food</strong> categories, in order to maintain some degree of c<strong>on</strong>sumerchoice.■ Voluntary fortificati<strong>on</strong> usually c<strong>on</strong>fers a higher level of c<strong>on</strong>sumer choice;however, this is not the main issue in many developing countries, wherepoverty remains the limiting factor to access to processed <strong>food</strong>s for themajority of the populati<strong>on</strong>.5. Food c<strong>on</strong>sumpti<strong>on</strong> patterns. Clearly, <strong>food</strong> c<strong>on</strong>sumpti<strong>on</strong> patterns, especially interms of the relative c<strong>on</strong>tributi<strong>on</strong>s of certain <strong>food</strong>s to the diet of the targetpopulati<strong>on</strong>, will have a bearing <strong>on</strong> the choice of mandatory or voluntary fortificati<strong>on</strong>.Linked to this factor is the issue of the technical suitability of thecandidate <strong>food</strong> as a vehicle for fortificati<strong>on</strong>.■ Foods c<strong>on</strong>sidered for mandatory fortificati<strong>on</strong> should be widely and regularlyc<strong>on</strong>sumed by the populati<strong>on</strong> group that the fortificati<strong>on</strong> is intended to benefit.In additi<strong>on</strong>, the fortificati<strong>on</strong> itself should be technically feasible.■ The likelihood of all at-risk c<strong>on</strong>sumers increasing their usual micr<strong>on</strong>utrientintake through voluntary fortificati<strong>on</strong> is lower than <strong>with</strong> mandatory fortificati<strong>on</strong>.However, the likelihood rises as the particular micr<strong>on</strong>utrient is added toa wider range of voluntarily-fortified <strong>food</strong>s, assuming they are accessible toc<strong>on</strong>sumers.37
PA RT I IEvaluating the publichealth significance ofmicr<strong>on</strong>utrient malnutriti<strong>on</strong>
Introducti<strong>on</strong>The chapters in Part II of these guidelines provide more detailed backgroundinformati<strong>on</strong> <strong>on</strong> the prevalence, causes and health c<strong>on</strong>sequences of variousmicr<strong>on</strong>utrient deficiencies, and review the available evidence regarding the benefitsof their c<strong>on</strong>trol. They are intended to assist planners not <strong>on</strong>ly in their evaluati<strong>on</strong>of the micr<strong>on</strong>utrient deficiency situati<strong>on</strong> in their own country, but alsoto assess the need for, and potential benefits of, <strong>food</strong> fortificati<strong>on</strong> <strong>with</strong> specificmicr<strong>on</strong>utrients.Chapter 3 looks at ir<strong>on</strong>, vitamin A and iodine deficiencies, which, owing totheir widespread occurrence globally, have received the most attenti<strong>on</strong> to date.A large amount of informati<strong>on</strong> is now available regarding the prevalence, thecauses and the c<strong>on</strong>trol of deficiencies in these three micr<strong>on</strong>utrients. Variousstudies <strong>on</strong> the efficacy and effectiveness of interventi<strong>on</strong>s to c<strong>on</strong>trol deficienciesin ir<strong>on</strong>, vitamin A and iodine, are briefly described here (and in the openingchapter of this document; see secti<strong>on</strong> 1.3), but are reviewed in greater depthelsewhere (73). Chapter 4 focuses <strong>on</strong> a range of other micr<strong>on</strong>utrients, which, incomparis<strong>on</strong>, have hitherto been somewhat neglected. Deficiencies in at leastsome of these “neglected” micr<strong>on</strong>utrients (i.e. in zinc, vitamins B 2 and B 12 ,niacin, vitamin D and calcium) are likely to be comm<strong>on</strong> throughout much ofthe developing world and am<strong>on</strong>g the poorest populati<strong>on</strong>s in the industrializednati<strong>on</strong>s. Fortificati<strong>on</strong> provides a means of lowering the prevalence of deficienciesin all of these micr<strong>on</strong>utrients, and their inclusi<strong>on</strong> in mass fortificati<strong>on</strong> programmes,in particular, could produce significant public health benefits. Sincethere is less informati<strong>on</strong> about these micr<strong>on</strong>utrient deficiencies in the literature,a c<strong>on</strong>certed effort has been made to summarize what is known about them inthese guidelines.In both chapters, micr<strong>on</strong>utrients are discussed in order of their perceivedpublic health significance, and in each case the recommended or the most comm<strong>on</strong>lyused biochemical status indicators are critically reviewed. For somemicr<strong>on</strong>utrients, however, biochemical data reflecting nutriti<strong>on</strong>al status will beinadequate for assessing the prevalence of deficiencies. Suggesti<strong>on</strong>s for dealing<strong>with</strong> this situati<strong>on</strong>, for example, by using <strong>food</strong> intake data to estimate the prevalenceof inadequate intakes, are provided in Part IV of these guidelines (seesecti<strong>on</strong> 7.3.2).41
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSOther than a low dietary intake, important causes of MNM include poorbioavailability from <strong>food</strong>s (especially for minerals), frequent infecti<strong>on</strong> <strong>with</strong> parasites,diarrhoea, and various malabsorpti<strong>on</strong> disorders. The presence of any ofthese risk factors can lead to an underestimati<strong>on</strong> of the prevalence of deficiencyin a populati<strong>on</strong> if this is calculated <strong>on</strong> the basis of micr<strong>on</strong>utrient intakes al<strong>on</strong>e.Risk factors for micr<strong>on</strong>utrient malnutriti<strong>on</strong>■ M<strong>on</strong>ot<strong>on</strong>ous diet resulting in low micr<strong>on</strong>utrient intake, and poor bioavailability, especiallyof minerals.■ Low intake of animal source <strong>food</strong>s.■ Low prevalence of breastfeeding.■ Low micr<strong>on</strong>utrient density of complementary <strong>food</strong>s.■ Increased physiological demands for growth during pregnancy and lactati<strong>on</strong>.■ Increased demand due to acute infecti<strong>on</strong> (especially if infecti<strong>on</strong> episodes are frequent),chr<strong>on</strong>ic infecti<strong>on</strong> (e.g. tuberculosis, malaria and HIV/AIDS) and disease (e.g.cancer).■ Poor general nutriti<strong>on</strong>al status, in particular, protein–energy malnutriti<strong>on</strong>.■ Malabsorpti<strong>on</strong> due to diarrhoea or the presence of intestinal parasites (e.g. Giardialamblia, hookworms).■ Increased excreti<strong>on</strong> (e.g. due to schistosomiasis).■ Seas<strong>on</strong>al variati<strong>on</strong>s in <strong>food</strong> availability, <strong>food</strong> shortages.■ Social deprivati<strong>on</strong>, illiteracy, low educati<strong>on</strong>.■ Poor ec<strong>on</strong>omic status and poverty.42
CHAPTER 3Ir<strong>on</strong>, vitamin A and iodine3.1 Ir<strong>on</strong> deficiency and anaemiaMost of the ir<strong>on</strong> in the human body is present in the erythrocytes as haemoglobin,where its main functi<strong>on</strong> is to carry oxygen from the lungs to the tissues.Ir<strong>on</strong> is also an important comp<strong>on</strong>ent of various enzyme systems, such as thecytochromes, which are involved in oxidative metabolism. It is stored in the liveras ferritin and as haemosiderin.Ir<strong>on</strong> deficiency is the most comm<strong>on</strong> and widespread nutriti<strong>on</strong>al disorderin the world, and is a public health problem in both industrialized and n<strong>on</strong>industrializedcountries. Ir<strong>on</strong> deficiency is the result of a l<strong>on</strong>g-term negative ir<strong>on</strong>balance; in its more severe stages, ir<strong>on</strong> deficiency causes anaemia. Anaemia isdefined as a low blood haemoglobin c<strong>on</strong>centrati<strong>on</strong>. Haemoglobin cut-off valuesthat indicate anaemia vary <strong>with</strong> physiological status (e.g. age, sex) and have beendefined for various populati<strong>on</strong> groups by WHO (1).3.1.1 Prevalence of deficiencyThe terms, “ir<strong>on</strong> deficiency” and “ir<strong>on</strong>-deficiency anaemia” are often used syn<strong>on</strong>ymouslyalthough they are in fact not the same c<strong>on</strong>diti<strong>on</strong>s. About 40% of theworld’s populati<strong>on</strong> (i.e. more than 2 billi<strong>on</strong> individuals) is thought to suffer fromanaemia, i.e. low blood haemoglobin (see Table 1.1). The mean prevalencesam<strong>on</strong>g specific populati<strong>on</strong> groups are estimated to be:— pregnant women, infants and children aged 1–2 years, 50%;— preschool-aged children, 25%;— schoolchildren, 40%;— adolescents, 30–55%;— n<strong>on</strong>-pregnant women, 35%.These average figures obscure the fact that ir<strong>on</strong> deficiency and ir<strong>on</strong>-deficiencyanaemia are even more prevalent in some parts of the world, especially in theIndian subc<strong>on</strong>tinent and in sub-Saharan Africa, where, for example, up to 90%of women become anaemic during pregnancy.43
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSThe prevalence of anaemia caused by ir<strong>on</strong> deficiency, usually referred to asir<strong>on</strong>-deficiency anaemia, is less certain because the specific indicators of ir<strong>on</strong>status, such as serum ferritin, transferrin saturati<strong>on</strong>, zinc protoporphyrin andserum transferrin receptors, are measured less often than blood haemoglobin(Table 3.1). Most indicators of ir<strong>on</strong> status – <strong>with</strong> the possible excepti<strong>on</strong> of serumtransferrin receptors – are also affected by the presence of infecti<strong>on</strong> and cantherefore be misleading (74). Indeed, every indicator listed in Table 3.1 has itsown set of limitati<strong>on</strong>s, and so ir<strong>on</strong> status is best assessed by a combinati<strong>on</strong> ofindicators (74).It is generally assumed that, <strong>on</strong> average, around 50% of the cases of anaemiaare due to ir<strong>on</strong> deficiency, as opposed to malaria (which causes anaemia becausethe malaria parasite destroys erythrocytes), the presence of infecti<strong>on</strong> or othernutrient deficiencies. However, the proporti<strong>on</strong> is probably higher in infants andpreschool-aged children than in older children or women (75), and is likely tovary by locati<strong>on</strong>. Although anaemia usually occurs when ir<strong>on</strong> stores are depleted,the prevalence of ir<strong>on</strong> deficiency will often be substantially higher than theprevalence of ir<strong>on</strong>-deficiency anaemia. However, in ir<strong>on</strong>-deficient populati<strong>on</strong>s<strong>with</strong> endemic malaria, the prevalence of anaemia will be greater than, or similarto, the prevalence of ir<strong>on</strong> deficiency (75). Furthermore, the use of serum ferritinas an indicator of ir<strong>on</strong> status may well overestimate the prevalence of ir<strong>on</strong>deficiency in malaria endemic areas; this is because serum ferritin levels are elevatedby the presence of infecti<strong>on</strong>s such as malaria (Table 3.1), and also thereas<strong>on</strong> why, traditi<strong>on</strong>ally, the cut-off level that defined ir<strong>on</strong> deficiency in individuals<strong>with</strong> malaria was higher (
3. IRON, VITAMIN A AND IODINETABLE 3.1Indicators for assessing ir<strong>on</strong> status at the populati<strong>on</strong> level aIndicator Sample Populati<strong>on</strong> group Cut-off to define CommentsdeficiencyMild SevereHaemoglobin b Blood Children 6–59 m<strong>on</strong>ths 110 g/l Not defined Blood haemoglobin is primarily an indicator of anaemia but canChildren 5–11 years 115 g/l provide useful informati<strong>on</strong> regarding ir<strong>on</strong> status, as follows:Children 12–14 years 120 g/l — An increase of at least 10 g/l in blood haemoglobin afterMen over 15 years 130 g/l 1 or 2 m<strong>on</strong>ths of ir<strong>on</strong> supplementati<strong>on</strong> is indicative ofWomen over 15 years 120 g/l baseline ir<strong>on</strong> deficiency.(n<strong>on</strong>-pregnant) — Where poor availability of dietary ir<strong>on</strong> is the main causePregnant women 110 g/l
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 3.1Indicators for assessing ir<strong>on</strong> status at the populati<strong>on</strong> level a (C<strong>on</strong>tinued)Indicator Sample Populati<strong>on</strong> group Cut-off to define CommentsdeficiencyMild SevereTransferrin Serum Can be applied to all Cut-off values vary Useful indicator of ir<strong>on</strong> status; not affected by infecti<strong>on</strong> andreceptors populati<strong>on</strong> groups <strong>with</strong> method used thus can be used in combinati<strong>on</strong> <strong>with</strong> measurement of serumferritin to c<strong>on</strong>firm deficiency in cases of infecti<strong>on</strong>.No universally agreed cut-offs; reference materials still need tobe standardized.Transferrin Serum Can be applied to all 70 µg/dl Elevated when ir<strong>on</strong> supply is inadequate for haem producti<strong>on</strong>.protoporphyrin (RBC) Over 5 years Normal >80 µg/dl Elevated in the presence of infecti<strong>on</strong>, lead pois<strong>on</strong>ing andhaemolytic anaemia.AGP, Alpha 1 acid glycoprotein; CRP, C-reactive protein; RBC, red blood cell.aEvery indicator of ir<strong>on</strong> status has limitati<strong>on</strong>s so the best way to assess ir<strong>on</strong> status is to use a combinati<strong>on</strong> of indicators.bHaemoglobin values for populati<strong>on</strong>s living at sea level require adjustment for selected variables, including altitude and tobacco c<strong>on</strong>sumpti<strong>on</strong>.Sources: reference (1,74).46
3. IRON, VITAMIN A AND IODINETABLE 3.2Criteria for assessing the public health severity ofanaemiaSeverity of the public healthproblemPrevalence of anaemia a(% of the populati<strong>on</strong>)N<strong>on</strong>e ≤4.9Mild 5.0–19.9Moderate 20.0–39.9Severe≥40aAnaemia is defined <strong>on</strong> the basis of blood haemoglobinc<strong>on</strong>centrati<strong>on</strong>s (see Table 3.1)Source: reference (1).TABLE 3.3Classificati<strong>on</strong> of usual diets according to their ir<strong>on</strong> bioavailabilityCategory Ir<strong>on</strong> bioavailability Dietary characteristics(%)Low 1–9 Simple, m<strong>on</strong>ot<strong>on</strong>ous diet based <strong>on</strong> cereals, roots ortubers, <strong>with</strong> negligible amounts of meat, fish, poultryor ascorbic acid-rich <strong>food</strong>s. Diet high in <strong>food</strong>s thatinhibit ir<strong>on</strong> absorpti<strong>on</strong> such as maize, beans, wholewheat flour and sorghum.Intermediate 10–15 Diet of cereals, roots or tubers, <strong>with</strong> some <strong>food</strong>s ofanimal origin (meat, fish or poultry) and/or c<strong>on</strong>tainingsome ascorbic acid (from fruits and vegetables).High >15 Diversified diet c<strong>on</strong>taining greater amounts of meat,fish, poultry and/or <strong>food</strong>s high in ascorbic acid.Sources: adapted from references (78,79).• periods of life when ir<strong>on</strong> requirements are especially high (i.e. growth andpregnancy);• heavy blood losses as a result of menstruati<strong>on</strong>, or parasite infecti<strong>on</strong>s such ashookworm, ascaris and schistosomiasis.As menti<strong>on</strong>ed above, acute or chr<strong>on</strong>ic infecti<strong>on</strong>s, including malaria, can alsolower haemoglobin c<strong>on</strong>centrati<strong>on</strong>s (76). The presence of other micr<strong>on</strong>utrientdeficiencies, especially of vitamins A and B 12 , folate and riboflavin, also increasesthe risk of anaemia (77).The dietary habits of a populati<strong>on</strong> group str<strong>on</strong>gly affect the bioavailability ofboth dietary ir<strong>on</strong> and added fortificant ir<strong>on</strong>. Estimates of the average bioavailabilityof ir<strong>on</strong> from different types of diets are provided in Table 3.3. Althoughthe efficiency of ir<strong>on</strong> absorpti<strong>on</strong> increases substantially as ir<strong>on</strong> stores become47
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSdepleted, the amount absorbed from <strong>food</strong>s, especially where diets are low inmeat, fish, fruit and vegetables, is not enough to prevent ir<strong>on</strong> deficiency in manywomen and children, especially in the developing world.3.1.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>The main c<strong>on</strong>sequences of ir<strong>on</strong> deficiency are anaemia, impaired cognitive andphysical performance, and increased maternal and child mortality (see Table1.2). Ir<strong>on</strong> deficiency has been shown to reduce physical endurance, even in theabsence of anaemia (80), and severe anaemia has been associated <strong>with</strong> anincreased risk of both maternal and child mortality (81,82). As indicated previously(see secti<strong>on</strong> 1.1), there is now substantial evidence to suggest that ir<strong>on</strong>supplementati<strong>on</strong> can reverse the adverse effects of ir<strong>on</strong> deficiency <strong>on</strong> workcapacity and productivity, and <strong>on</strong> pregnancy outcome and child development(14–16). In a study in the United States, for example, ir<strong>on</strong> supplementati<strong>on</strong>during pregnancy reduced the number of preterm deliveries and lowbirth-weightinfants (83).Improving ir<strong>on</strong> status may have other, but as yet poorly appreciated, benefitsfor health, most noticeably <strong>with</strong> respect to the utilizati<strong>on</strong> of vitamin A and iodine.That vitamin A (retinol) is mobilized from the liver by an ir<strong>on</strong>-dependentenzyme is well-established fact, but more recently, experimental studies havesuggested that in cases of ir<strong>on</strong> deficiency the vitamin is trapped in the liverand thus may be less accessible to other tissues and organs (84). Furthermore,ir<strong>on</strong> supplementati<strong>on</strong> of ir<strong>on</strong>-deficient individuals increased plasma retinolin some studies through mechanisms that are as yet incompletely understood(85). Similarly, ir<strong>on</strong> is required by the enzymes that synthesize thyroxine, andthus a low ir<strong>on</strong> status may have implicati<strong>on</strong>s for iodine metabolism. Studies inCôte d’Ivoire have dem<strong>on</strong>strated that recovery from goitre after iodine treatmentis slower in ir<strong>on</strong>-deficient individuals (86). In a populati<strong>on</strong> of children <strong>with</strong> ahigh prevalence of anaemia and goitre, ir<strong>on</strong> supplementati<strong>on</strong> improved theresp<strong>on</strong>se to iodized oil or iodized salt (87) (see also secti<strong>on</strong> 1.3.2.3). Onthe basis of the above findings, it is reas<strong>on</strong>able to assume that improvementsin the ir<strong>on</strong> status of a populati<strong>on</strong> may well have benefits for vitamin A and iodinemetabolism.3.2 Vitamin AVitamin A is an essential nutrient that is required in small amounts by humansfor the normal functi<strong>on</strong>ing of the visual system, the maintenance of cell functi<strong>on</strong>for growth, epithelial cellular integrity, immune functi<strong>on</strong> and reproducti<strong>on</strong>.Dietary requirements for vitamin A are normally provided as a mixture of preformedvitamin A (retinol), which is present in animal source <strong>food</strong>s, and provitaminA carotenoids, which are derived from <strong>food</strong>s of vegetable origin and which48
3. IRON, VITAMIN A AND IODINEhave to be c<strong>on</strong>verted into retinol by tissues such as the intestinal mucosa andthe liver in order to be utilized by cells.Aside from the clinical ocular signs, i.e. night blindness and xerophthalmia,symptoms of vitamin A deficiency (VAD) are largely n<strong>on</strong>-specific. Nevertheless,accumulated evidence suggests that VAD is an important determinant ofchild survival and safe motherhood (see secti<strong>on</strong> 3.2.3). The n<strong>on</strong>-specificityof symptoms, however, means that, in the absence of biochemical measures ofvitamin A status, it is difficult to attribute n<strong>on</strong>-ocular symptoms to VAD and italso complicates the definiti<strong>on</strong> of VAD.With these c<strong>on</strong>siderati<strong>on</strong>s in mind,WHOhas defined VAD as tissue c<strong>on</strong>centrati<strong>on</strong>s of vitamin A low enough to haveadverse health c<strong>on</strong>sequences, even if there is no evidence of clinical xerophthalmia(5). In more recent years, the term “vitamin A deficiency disorders” hasbeen coined to reflect the diversity of adverse outcomes caused by vitamin Adeficiency (88).3.2.1 Prevalence of deficiencyAs vitamin A deficiency affects visual functi<strong>on</strong>, indicators of vitamin A statushave traditi<strong>on</strong>ally relied <strong>on</strong> changes in the eye, specifically night blindness andxerophthalmia (5) (Table 3.4).Worldwide, about 3 milli<strong>on</strong> preschool-aged childrenpresent ocular signs of VAD (3). Vitamin A deficiency is, however, morecomm<strong>on</strong>ly assessed using serum or plasma retinol levels. WHO estimates that254 milli<strong>on</strong> preschool-aged children throughout the world have low serumretinol levels and can therefore be c<strong>on</strong>sidered to be clinically or subclinicallyvitamin A deficient (3). In the developing world, prevalence rates in this agegroup range from 15% up to as high as 60%, <strong>with</strong> Latin America, the EasternMediterranean and the Western Pacific being at the low end of this range, andAfrica and South-East Asia occupying the high end (3,89) (see also Table 1.1).The prevalence of night blindness is also high am<strong>on</strong>g pregnant women in manypoor regi<strong>on</strong>s of the world, <strong>with</strong> rates varying between 8% and 24% (89). Nightblindness tends to be accompanied by a high prevalence of low c<strong>on</strong>centrati<strong>on</strong>sof retinol in breast milk (
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 3.4Indicators for assessing vitamin A status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> group Cut-off to define CommentsdeficiencyMild SeverePrevalence of Clinical Children 6–71 >1% >5%night examinati<strong>on</strong> m<strong>on</strong>thsblindness Pregnant women >5% Not defined Night blindness prevalence is assessed by interview about(%) reported occurrence during last pregnancy.Retinol Serum or Preschool-age 0.35–0.7µmol/l
3. IRON, VITAMIN A AND IODINETABLE 3.5Criteria for assessing the public health severity of vitamin A deficiencyIndicator Populati<strong>on</strong> group Prevalence indicating a public healthproblem (% of the populati<strong>on</strong>)Night blindness Pregnant women >5Night blindness Children 24–71 m<strong>on</strong>ths >1Bitot’s spots Children 24–71 m<strong>on</strong>ths >0.5Serum retinol
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSimprovement of vitamin A status, whether by supplementati<strong>on</strong> or fortificati<strong>on</strong>,decreased all-cause mortality in children aged between 6 m<strong>on</strong>ths and 5 years by23% (12).In additi<strong>on</strong> to causing night blindness, vitamin A deficiency is probably animportant c<strong>on</strong>tributor to maternal mortality and other poor outcomes in pregnancyand lactati<strong>on</strong>. According to the results of <strong>on</strong>e study, in which vitamin A-deficient pregnant women received vitamin A or β-carotene supplements atdoses equivalent to their weekly requirement for the vitamin, maternal mortalitywas reduced by 40% and 49%, respectively, relative to a c<strong>on</strong>trol group (97).Other studies have shown night blindness to be a risk factor for maternal mortalityand morbidity: in Nepal, for example, the death rate from infecti<strong>on</strong>s wasabout five times higher am<strong>on</strong>g unsupplemented pregnant women who reportednight blindness compared <strong>with</strong> those who did not (98). Vitamin A deficiencyalso increases vulnerability to other disorders, such as ir<strong>on</strong> deficiency (see secti<strong>on</strong>3.1.3). Providing an ir<strong>on</strong> supplement <strong>with</strong> vitamin A to pregnant women inInd<strong>on</strong>esia increased haemoglobin c<strong>on</strong>centrati<strong>on</strong>s by approximately 10g/l morethan did supplementati<strong>on</strong> <strong>with</strong> ir<strong>on</strong> al<strong>on</strong>e (99).3.3 IodineIodine is present in the body in minute amounts, mainly in the thyroid gland.Its <strong>on</strong>ly c<strong>on</strong>firmed role is in the synthesis of thyroid horm<strong>on</strong>es. Iodine deficiencyis a major public health problem for populati<strong>on</strong>s throughout the world, but particularlyfor young children and pregnant women, and in some settings representsa significant threat to nati<strong>on</strong>al social and ec<strong>on</strong>omic development. The mostdevastating outcome of iodine deficiency is mental retardati<strong>on</strong>: it is currently<strong>on</strong>e of the world’s main causes of preventable cognitive impairment. This is theprimary motivati<strong>on</strong> behind the current worldwide drive to eliminate iodine deficiencydisorders (IDD).3.3.1 Prevalence of deficiencyThe recommended indicators for assessing the extent of iodine deficiency <strong>with</strong>ina populati<strong>on</strong> are median urinary iodine and total goitre prevalence (Table 3.6).According to generally accepted criteria, iodine deficiency is a public healthproblem in populati<strong>on</strong>s where the median urinary iodine c<strong>on</strong>centrati<strong>on</strong> is below100µg/l, or in areas where goitre is endemic, that is to say, where more than 5%of children aged 6–12 years have goitre (Table 3.7).As the median urinary iodine c<strong>on</strong>centrati<strong>on</strong> reflects current iodine intake andresp<strong>on</strong>ds relatively rapidly to the correcti<strong>on</strong> of iodine deficiency, it is usually thepreferred indicator for m<strong>on</strong>itoring the impact of interventi<strong>on</strong>s for IDD c<strong>on</strong>trol.An expanded set of indicators for assessing nati<strong>on</strong>al progress towards the goalof the sustainable eliminati<strong>on</strong> of IDDs is given in Annex A. This indicator set,52
3. IRON, VITAMIN A AND IODINETABLE 3.6Indicators for assessing iodine status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> group Cut-off to define CommentsdeficiencyMild SevereIodine Urine Children Median Median Recommended indicator for m<strong>on</strong>itoring or evaluating iodine6–12 years 30% Reflects past or current thyroid dysfuncti<strong>on</strong> and can beprevalence examinati<strong>on</strong> 6–12 years measured by clinical examinati<strong>on</strong> or by ultras<strong>on</strong>ography.Not recommended for m<strong>on</strong>itoring the impact of interventi<strong>on</strong>s asgoitre resp<strong>on</strong>se to iodine status correcti<strong>on</strong> is delayed.Source: reference (6).53
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 3.7Criteria for assessing the public health severity of iodine deficiencySeverity of publichealth problemIndicatorMedian urinary iodine (µg/l) Total goitre prevalence (%)Mild 50–99 5.0–19.9Moderate 20–49 20–29.9Severe 30Source: reference (6).which has been recommended by WHO, relates not just to the populati<strong>on</strong>’siodine status (as measured by urinary c<strong>on</strong>centrati<strong>on</strong>s) but includes various programmaticindicators which measure the sustainability of the salt iodizati<strong>on</strong> programmeitself.According to recent WHO estimates, some 1989 milli<strong>on</strong> people have inadequateiodine nutriti<strong>on</strong> (2). The WHO regi<strong>on</strong>s, ranked by the absolute numberof people affected are, in decreasing order of magnitude, South-East Asia,Europe, the Western Pacific, Africa, the Eastern Mediterranean and theAmericas (see Table 1.1). In some parts of the world, for example, in parts ofeastern and western Europe, iodine deficiency, in its subclinical form, is reemerging,having previously been eliminated. This underscores the need tosustain efforts to c<strong>on</strong>trol iodine deficiency <strong>on</strong> a global scale.3.3.2 Risk factors for deficiencyThe main factor resp<strong>on</strong>sible for the development of iodine deficiency is alow dietary supply of iodine (100). This tends to occur in populati<strong>on</strong>s livingin areas where the soil has been deprived of iodine as the result of past glaciati<strong>on</strong>,and subsequently, because of the leaching effects of snow, water and heavyrainfall.Iodine deficiency is exacerbated by a high c<strong>on</strong>sumpti<strong>on</strong> of natural goitrogensthat are present in some staple <strong>food</strong>s such as cassava. The antithyroid acti<strong>on</strong> ofgoitrogens is related to the presence of thiocyanate which inhibits thyroid iodidetransport and, at higher doses, competes <strong>with</strong> iodide in the synthesis of thyroidhorm<strong>on</strong>es (101). Goitrogenicity is determined by the balance between thedietary supply of iodine and thiocyanate: goitre develops when the urinary iodine(µg): thiocyanate (mg) ratio falls below 3.3.3.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>Iodine deficiency is associated <strong>with</strong> a large range of abnormalities, groupedunder the heading of “iodine deficiency disorders”, that reflect thyroid54
3. IRON, VITAMIN A AND IODINETABLE 3.8The spectrum of iodine deficiency disordersFetusNe<strong>on</strong>ateChild, adolescentand adultAborti<strong>on</strong>sStillbirthsC<strong>on</strong>genital abnormalitiesIncreased infant mortalityCognitive impairment and neurological disorders, including endemiccretinism and endemic mental retardati<strong>on</strong>HypothyroidismIncreased susceptibility of the thyroid gland to nuclear radiati<strong>on</strong>HypothyroidismGoitreRetarded physical development in child and adolescentImpaired mental functi<strong>on</strong>Decreased fertilityIodine-induced hyperthyroidism in adultsIncreased susceptibility of the thyroid gland to nuclear radiati<strong>on</strong>Sp<strong>on</strong>taneous hyperthyroidism in the elderlyGoitre <strong>with</strong> its complicati<strong>on</strong>sSource: adapted from reference (9).dysfuncti<strong>on</strong> (9). Goitre and cretinism are the most visible manifestati<strong>on</strong>s ofiodine deficiency; others include hypothyroidism, decreased fertility rate,increased perinatal death and infant mortality (Table 3.8).When iodine intake is abnormally low, an adequate producti<strong>on</strong> of thyroid horm<strong>on</strong>esmay still be achieved by increased secreti<strong>on</strong> of thyroid stimulatinghorm<strong>on</strong>e (TSH). However, a prol<strong>on</strong>ged stimulati<strong>on</strong> of the thyroid gland by TSHwill result in goitre. This c<strong>on</strong>diti<strong>on</strong> is indicative of thyroid hyperplasia, whichoccurs because of the thyroid’s inability to synthesize sufficient thyroidhorm<strong>on</strong>es.Irreversible mental retardati<strong>on</strong> is the most serious disorder induced by iodinedeficiency (9,102,103). A deficit in iodine resulting in thyroid failure duringthe critical period of brain development, that is, from fetal life up to the thirdm<strong>on</strong>th after birth, will result in irreversible alterati<strong>on</strong>s in brain functi<strong>on</strong>(104,105). In areas of severe endemic iodine deficiency, cretinism may affect upto 5–15% of the populati<strong>on</strong>. Some individuals living in regi<strong>on</strong>s of mild or moderateiodine deficiency exhibit neurological and intellectual deficits that aresimilar to, but less marked, than those found in overt cretins. A meta-analysis of19 studies c<strong>on</strong>ducted in regi<strong>on</strong>s of severe deficiency showed that iodine deficiencyis resp<strong>on</strong>sible for a mean IQ loss of 13.5 points am<strong>on</strong>g affected populati<strong>on</strong>s(104).55
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSCorrecti<strong>on</strong> of iodine deficiency, when carried out at the right time, reducesor eliminates all c<strong>on</strong>sequences of iodine deficiency. The validity of this statementis borne out by the sharp reducti<strong>on</strong> in the incidence of IDD that is c<strong>on</strong>sistentlyobserved when iodine is added to the diet (see secti<strong>on</strong> 1.3), and the recurrenceof IDD when an effective IDD c<strong>on</strong>trol programme is interrupted in a previouslyiodine-deficient populati<strong>on</strong> (106).56
CHAPTER 4Zinc, folate, vitamin B 12 and other Bvitamins, vitamin C, vitamin D, calcium,selenium and fluoride4.1 ZincZinc is an essential comp<strong>on</strong>ent of a large number of enzymes, and plays a centralrole in cellular growth and differentiati<strong>on</strong> in tissues that have a rapid differentiati<strong>on</strong>and turnover, including those of the immune system and those in the gastrointestinaltract. The positive impact of zinc supplementati<strong>on</strong> <strong>on</strong> the growthof some stunted children, and <strong>on</strong> the prevalence of selected childhood diseasessuch as diarrhoea, suggests that zinc deficiency is likely to be a significant publichealth problem, especially in developing countries. However, the extent of zincdeficiency worldwide is not well documented. All populati<strong>on</strong> age groups are atrisk of zinc deficiency, but infants and young children are probably the mostvulnerable. Pregnant and lactating women are also likely to be very susceptibleto zinc deficiency, and there is an urgent need for more informati<strong>on</strong> <strong>on</strong> the implicati<strong>on</strong>sof low zinc status in these particular populati<strong>on</strong> groups (107,108).4.1.1 Prevalence of deficiencyThe lack of reliable and widely accepted indicators of zinc status of adequatesensitivity means that the global prevalence of zinc deficiency is uncertain.Thoseindicators that are available, such as zinc c<strong>on</strong>centrati<strong>on</strong> in plasma and hair (seeTable 4.1), detect changes in zinc status <strong>on</strong>ly in cases of severe deficiency, andmay fail to detect marginal deficiency.As suggested above, there are, however, several good reas<strong>on</strong>s to suspect thatzinc deficiency is comm<strong>on</strong>, especially in infants and children. Firstly, a highprevalence of low plasma zinc, which is a reas<strong>on</strong>able indicator of relatively severedepleti<strong>on</strong>, has been observed in some populati<strong>on</strong> groups. Sec<strong>on</strong>dly, several randomizedc<strong>on</strong>trol trials have dem<strong>on</strong>strated that stunted children, and/or those<strong>with</strong> low plasma zinc, resp<strong>on</strong>d positively to zinc supplementati<strong>on</strong>, a findingthat suggests that zinc deficiency was a limiting factor in their growth. Growthstunting affects about a third of children in less wealthy regi<strong>on</strong>s of the worldand is very comm<strong>on</strong> in settings where diets are of poor quality. This is nottoo say that zinc deficiency affects up to <strong>on</strong>e third of children in the developingworld since zinc deficiency is <strong>on</strong>ly but <strong>on</strong>e of several possible causes of growthstunting.57
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 4.1Indicators for assessing zinc status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> group Cut-off to define CommentsdeficiencyZinc Serum or Applies to all
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDE4.1.2 Risk factors for deficiencyThe central role of zinc in cell divisi<strong>on</strong>, protein synthesis and growth means thatan adequate supply is especially important for infants, and pregnant and lactatingwomen. Principal risk factors for zinc deficiency include diets low in zinc orhigh in phytates, malabsorpti<strong>on</strong> disorders (including the presence of intestinalparasites and diarrhoea), impaired utilizati<strong>on</strong> of zinc and genetic diseases (e.g.acrodermatitis enteropathica, sickle-cell anaemia) (Table 1.2).The bioavailability of zinc is dependent <strong>on</strong> dietary compositi<strong>on</strong>, in particular,<strong>on</strong> the proporti<strong>on</strong> of high-phytate <strong>food</strong>s in the diet (i.e. selected cereals andlegumes). The molar ratio of phytate:zinc in meals or diets provides a usefulmeasure of zinc bioavailability. At high ratios (i.e. above 15 : 1), zinc absorpti<strong>on</strong>from <strong>food</strong> is low, that is to say, less than 15% (110,111). The inclusi<strong>on</strong> of animalproteins can improve the total zinc intake and the efficiency of zinc absorpti<strong>on</strong>from a phytate-c<strong>on</strong>taining diet (112). For instance, the additi<strong>on</strong> of animal source<strong>food</strong>s to a diet based <strong>on</strong> rice and wheat approximately doubled the amount ofzinc that was absorbed by young Chinese women (113). Using data obtainedfrom experimental zinc absorpti<strong>on</strong> studies, various criteria have been developedto differentiate between diets likely to have high, moderate and low zinc bioavailability;these are summarized in Table 4.2.The extent to which the presence of phytates inhibits the absorpti<strong>on</strong> of zincis not precisely known at the present time. It is interesting to note that severalstudies have shown that zinc absorpti<strong>on</strong> from some legume-based diets is comparableto that from a diet based <strong>on</strong> animal products, despite the relatively highphytate c<strong>on</strong>tent of the former (112,114), and that in adult women, approximately30% of dietary zinc is absorbed across a wide range of different diets(93). In a c<strong>on</strong>trolled experiment, infants absorbed nearly 45% of the zinc froma wheat-soy complementary <strong>food</strong>, regardless of whether it c<strong>on</strong>tained 0.77% or0.3% phytic acid (115). In Malawi, 24% of the zinc was absorbed from highphytatemaize meals c<strong>on</strong>sumed by children, again a relatively high proporti<strong>on</strong>given the phytate c<strong>on</strong>tent (116).Competitive interacti<strong>on</strong>s can occur between zinc and other minerals that havesimilar physical and chemical properties, such as ir<strong>on</strong> and copper. When presentin large amounts (e.g. in the form of supplements) or in aqueous soluti<strong>on</strong>, theseminerals reduce zinc absorpti<strong>on</strong>. However, at the levels present in the usual dietand in fortified <strong>food</strong>s, zinc absorpti<strong>on</strong> is not generally affected (93). On the otherhand, high levels of dietary calcium (i.e. >1g per day), which might be c<strong>on</strong>sumedby some individuals, can inhibit zinc absorpti<strong>on</strong>, especially in the presenceof phytates. The degree of impairment varies depending <strong>on</strong> the type of dietand the source of the calcium (93). Unlike ir<strong>on</strong>, zinc absorpti<strong>on</strong> is neither inhibitedby phenolic compounds, nor enhanced by vitamin C.59
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 4.2Classificati<strong>on</strong> of usual diets according to the potential bioavailability of theirzinc c<strong>on</strong>tentBioavailability aMain dietary characteristicsHighRefined diets low in cereal fibre, low in phytic acid c<strong>on</strong>tent, and <strong>with</strong> aphytate :zinc molar ratio 1g Ca 2+ /day).Bioavailability of zinc improves when the diet includes animal proteinsources (including milk).Low Diets high in unrefined, unfermented and ungerminated cereal grains b ,especially when fortified <strong>with</strong> inorganic calcium salts and when intake ofanimal protein is negligible.Phytate: zinc molar ratio of total diet exceeds 15 c .High-phytate soy protein products c<strong>on</strong>stitute the primary protein source.Diets in which, singly or collectively, approximately 50% of the energy intakeis from the following high-phytate <strong>food</strong>s: high-extracti<strong>on</strong>-rate (≥90%)wheat, rice, maize, grains and flours, oatmeal, and millet; chapatti floursand tanok; and sorghum, cowpeas, pige<strong>on</strong> peas, grams, kidney beans,blackeyed beans, and groundnut flours.High intakes of inorganic calcium salts (>1g Ca 2+ /day), either assupplements or as adventitious c<strong>on</strong>taminants (e.g. from calcareousgeophagia), potentiate the inhibitory effects; low intakes of animal proteinexacerbate these effects.aAt intakes adequate to meet the average normative requirements for absorbed zinc the threebioavailability levels corresp<strong>on</strong>d to 50%, 30% and 15% absorpti<strong>on</strong>. With higher zinc intakes,the fracti<strong>on</strong>al absorpti<strong>on</strong> is lower.bGerminati<strong>on</strong> of such grains or fermentati<strong>on</strong> of many flours can reduce antag<strong>on</strong>istic potency;if cereal grains have been germinated then the diet should then be classified as havingmoderate zinc bioavailability.cVegetable diets <strong>with</strong> phytate:zinc ratios >30 are not unknown; for such diets, an assumpti<strong>on</strong>of 10% bioavailability of zinc or less may be justified, especially if the intake of protein is low,or the intake of inorganic calcium salts is excessive, or both.Source: reference (93).The influence of all of the above-menti<strong>on</strong>ed risk factors for zinc deficiencyis difficult to integrate in any coherent way. In particular, further research isneeded to evaluate the bioavailability of zinc from usual diets in developingcountries and to better understand the relati<strong>on</strong>ship between dietary patterns andzinc supply.60
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDE4.1.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>Zinc deficiency is often hard to identify as its clinical manifestati<strong>on</strong>s are largelyn<strong>on</strong>-specific (Table 1.2). The symptoms of severe deficiency include dermatitis,retarded growth, diarrhoea, mental disturbances and recurrent infecti<strong>on</strong>s.Moderate and mild deficiencies are even more difficult to diagnose, not <strong>on</strong>lybecause they are characterized by a diversity of symptoms, but also <strong>on</strong> accountof the fact that there are no suitable biomarkers of zinc deficiency (117).In children, impaired growth (stunting) is <strong>on</strong>e of the possible c<strong>on</strong>sequencesof zinc deficiency. Zinc supplementati<strong>on</strong> trials c<strong>on</strong>ducted over the last fewdecades in children from developing countries have clearly dem<strong>on</strong>strated thepositive benefits of improved zinc status, including improved growth rates andreducti<strong>on</strong>s in the incidence of various infectious diseases (17,18,118). Forexample, a meta-analysis of randomized c<strong>on</strong>trolled supplementati<strong>on</strong> trialsreported an 18% decrease in diarrhoea incidence, a 25% reducti<strong>on</strong> in diarrhoeaprevalence, and a 41% fall in the incidence of pneum<strong>on</strong>ia (18). Zinc supplementati<strong>on</strong>also led to fewer episodes of malaria and fewer clinic visits due tocomplicati<strong>on</strong>s of malaria in Papua New Guinea (118), but not in Burkina Faso(119).The effect of maternal zinc status <strong>on</strong> pregnancy outcomes is unclear at thepresent time (120). Although severe zinc deficiency has been associated <strong>with</strong>poor maternal pregnancy outcomes (121), studies involving moderate deficiencyhave proved inc<strong>on</strong>clusive (122). Maternal zinc supplementati<strong>on</strong> in Peruimproved fetal neurobehavioral development (123), but had no effect <strong>on</strong> size atbirth or pregnancy durati<strong>on</strong> (124). In India, zinc supplements helped to reducemortality am<strong>on</strong>g low-birth-weight infants (125). Interestingly, the zinc c<strong>on</strong>tentof breast milk has not been shown to correlate <strong>with</strong> maternal zinc intake andappears to be unaffected by supplementati<strong>on</strong> (126,127).4.2 FolateFolate (vitamin B 9 ) plays a central role in the synthesis and methylati<strong>on</strong> ofnucleotides that intervene in cell multiplicati<strong>on</strong> and tissue growth. Its role inprotein synthesis and metabolism is closely interrelated to that of vitamin B 12 .The combinati<strong>on</strong> of severe folate deficiency and vitamin B 12 deficiency can resultin megaloblastic anaemia. Low intakes of folate are also associated <strong>with</strong> a higherrisk of giving birth to infants <strong>with</strong> neural tube defects and possibly other birthdefects, and <strong>with</strong> an increased risk of cardiovascular diseases, cancer andimpaired cognitive functi<strong>on</strong> in adults.4.2.1 Prevalence of deficiencySerum folate is a good indicator of recent dietary folate intake, and the mostwidely used method of assessing folate status (128). Erythrocyte folate is,61
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTShowever, the better indicator of l<strong>on</strong>g-term status and of tissue folate stores. Elevatedplasma homocysteine c<strong>on</strong>centrati<strong>on</strong>s are a str<strong>on</strong>g predictor of inadequatefolate status. However, other vitamin deficiencies (e.g. vitamins B 2 ,B 6 and B 12 )also increase homocysteine values. Indicators of folate status are summarized inTable 4.3 (93,128,129).The global prevalence of folate deficiency is uncertain, owing to a lack of data(130). Only a few countries have nati<strong>on</strong>al or even regi<strong>on</strong>al biochemical data <strong>on</strong>folate status. Furthermore, efforts to compare usual dietary intakes <strong>with</strong> estimatedrequirements (an alternative means of assessing the likely prevalence ofdeficiency in a populati<strong>on</strong>) are hampered by difficulties in measuring the folatec<strong>on</strong>tent of <strong>food</strong>s.TABLE 4.3Indicators for assessing folate (vitamin B 9 ) status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> Cut-off to define CommentsgroupdeficiencyFolate Serum Applies to all
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEFolate deficiency tends to be more prevalent in populati<strong>on</strong>s that have a highintake of refined cereals (which are low in folate) and a low intake of leafy greensand fruits (which are high in folate). Dietary surveys in India show that peopleeating predominantly cereal-based diets <strong>on</strong>ly c<strong>on</strong>sume about 75µg folate perday (131). Prior to the introducti<strong>on</strong> of mandatory wheat flour fortificati<strong>on</strong> <strong>with</strong>folic acid in 1998, about 15% of adult women in the United States were believedto have low serum and/or erythrocyte folate levels. Similarly, in Chile, where thec<strong>on</strong>sumpti<strong>on</strong> of white wheat flour is high, low serum and erythrocyte folate c<strong>on</strong>centrati<strong>on</strong>swere comm<strong>on</strong> before the fortificati<strong>on</strong> of flour <strong>with</strong> folic acid (132).In c<strong>on</strong>trast, low plasma values are rare in countries such as Guatemala, Mexicoand Thailand (77) where diets typically c<strong>on</strong>tain a higher proporti<strong>on</strong> of fruits andvegetables. For instance, few whole blood samples from the Mexican Nati<strong>on</strong>alNutriti<strong>on</strong> Survey were low in folate, <strong>with</strong> the excepti<strong>on</strong> of those of childrenunder 4 years of age, in which the prevalence of low blood folate was about 10%(133). Because of the high folate c<strong>on</strong>tent of certain legumes, fruits and vegetablesrelative to refined cereals, it is possible that populati<strong>on</strong>s in some developingcountries c<strong>on</strong>sume more folate than those in industrialized countries.Similarly, a study of pregnant women in Germany found that those who arelacto-ovo vegetarians (i.e. milk and egg c<strong>on</strong>sumers) or low meat c<strong>on</strong>sumers hadhigher levels of erythrocyte folate than the n<strong>on</strong>-vegetarians; this was attributedto the fact that the lacto-ovo vegetarians were c<strong>on</strong>suming proporti<strong>on</strong>ately morefolate-rich vegetables than their n<strong>on</strong>-vegetarian counterparts (134).4.2.2 Risk factors for deficiencyThe main sources of dietary folate are leafy green vegetables, fruits, yeast andliver. A low intake of these <strong>food</strong>s combined <strong>with</strong> a relatively high intake of refinedcereals thus increases the risk for folate deficiency. Malabsorpti<strong>on</strong> c<strong>on</strong>diti<strong>on</strong>s,infecti<strong>on</strong> <strong>with</strong> Giardia lamblia, bacterial overgrowth, genetic disorders (of folicacid metabolism) and chr<strong>on</strong>ic alcoholism are also risk factors for folate deficiency(see Table 1.2).4.2.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>Possible health c<strong>on</strong>sequences of a low folate status, which include megaloblasticanaemia, are summarized in Table 1.2. Folic acid has l<strong>on</strong>g been included inir<strong>on</strong> supplements provided to pregnant women in developing countries, despiterather limited evidence from Africa and India that folic acid reduces the risk ofmegaloblastic anaemia. In fact, there is little evidence to suggest that giving folicacid <strong>with</strong> ir<strong>on</strong> is any better at preventing anaemia than providing ir<strong>on</strong> al<strong>on</strong>e(77,135).Randomized trials c<strong>on</strong>ducted in China (136), the United States (137) and invarious other locati<strong>on</strong>s have c<strong>on</strong>sistently shown that folic acid supplements taken63
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSbefore and during the first 28 days after c<strong>on</strong>cepti<strong>on</strong> reduce the risk of womengiving birth to an infant <strong>with</strong> a neural tube defect (138). Neural tube defectsare serious malformati<strong>on</strong>s resulting in death or major lifel<strong>on</strong>g disability in survivors;worldwide, an estimated 300 000 or more ne<strong>on</strong>ates are affected each year(139). Studies have also dem<strong>on</strong>strated that folic acid supplementati<strong>on</strong> benefitssome women who have an abnormal folate metabolism because of a geneticdefect that affects their ability to utilize folate (140). Moreover, an analysis ofdata from different trials in which micr<strong>on</strong>utrients were provided during pregnancyfound folic acid to be the <strong>on</strong>ly micr<strong>on</strong>utrient that was associated <strong>with</strong> areduced risk of preterm delivery (141).Several interventi<strong>on</strong> trials have dem<strong>on</strong>strated that folic acid fortificati<strong>on</strong>lowers plasma homocysteine, even in populati<strong>on</strong>s <strong>with</strong> a relatively low prevalenceof folate deficiency (49). Several lines of evidence indicate that evenmoderately elevated plasma homocysteine is an independent risk factor forcardiovascular disease (142) and stroke (143), both leading causes of death inmany countries. While there is still some c<strong>on</strong>troversy c<strong>on</strong>cerning the directi<strong>on</strong>of causality (144), a comparis<strong>on</strong> of the results of genetic and prospectiveepidemiological studies, which would be expected to have different biases,str<strong>on</strong>gly points to a direct causal pathway leading from elevated homocysteineto cardiovascular disease (145). Higher plasma homocysteine levels are alsoassociated in industrialized countries <strong>with</strong> a higher risk of impaired cognitivefuncti<strong>on</strong> in adults (146), and many abnormal pregnancy outcomes, includingeclampsia and premature delivery, and other birth defects such as orofacial cleftpalate and heart defects. However, the evidence for the benefits of supplementati<strong>on</strong>for these c<strong>on</strong>diti<strong>on</strong>s is not as str<strong>on</strong>g than that linking supplementati<strong>on</strong> topreventi<strong>on</strong> of neural tube defects (147).The additi<strong>on</strong> of folic acid to enriched grain products in the United States, apractice which, as menti<strong>on</strong>ed above, was introduced in 1998, has since produceda substantial increase in average blood folate levels am<strong>on</strong>g women of childbearingage (148).This has resulted in the virtual eliminati<strong>on</strong> of low serum folate(149) and the lowering of plasma homocysteine in the populati<strong>on</strong> at large (49).The level of folic acid added (140µg/100 g flour) is unlikely to bring total folateintakes above the Tolerable Upper Intake Level (UL) of 1 000µg per day in anylife stage or gender group (128), or to exacerbate or obscure problems causedby vitamin B 12 deficiency (see secti<strong>on</strong> 4.3).4.3 Vitamin B 12Vitamin B 12 (cobalamin) is a cofactor in the synthesis of an essential amino acid,methi<strong>on</strong>ine. Its metabolic role is closely linked to that of folate in that <strong>on</strong>e of thevitamin B 12 -dependent enzymes, methi<strong>on</strong>ine synthase, is vital to the functi<strong>on</strong>ingof the methylati<strong>on</strong> cycle in which 5-methyltetrahydrofolate acts as a source of64
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEmethyl d<strong>on</strong>or groups which are necessary for cell metabolism and survival.Deficiency of this vitamin can thus impair the utilizati<strong>on</strong> of folate and causesneurological deteriorati<strong>on</strong>, megaloblastic anaemia, elevated plasma homocysteineand possibly, impaired immune functi<strong>on</strong>. In infants and young children itcan cause severe developmental delays.4.3.1 Prevalence of deficiencyVitamin B 12 status is usually assessed by measuring c<strong>on</strong>centrati<strong>on</strong>s in plasma orserum (Table 4.4) (93,128,129) Although elevated urinary and plasma methylmal<strong>on</strong>icacid (MMA) levels are more specific, and often more sensitive, indicatorsof vitamin B 12 deficiency, MMA c<strong>on</strong>centrati<strong>on</strong>s are more difficult andexpensive to measure than those of vitamin B 12 . Elevated homocysteine is a goodpredictor of vitamin B 12 status.TABLE 4.4Indicators for assessing vitamin B 12 (cobalamin) status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> Cut-off to define CommentsgroupdeficiencyVitamin B 12 Serum or Applies to all
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSVariability in the plasma levels used to define vitamin B 12 deficiency (seeTable 4.4) make the results of the few studies of its prevalence difficult to generalize.Moreover, there is no clear evidence that vitamin B 12 deficiency varies<strong>with</strong> countries or regi<strong>on</strong>s. In countries where vitamin B 12 deficiency has beenassessed at the nati<strong>on</strong>al level, low serum vitamin B 12 c<strong>on</strong>centrati<strong>on</strong>s were prevalent,i.e. in Venezuela (11–12% in preschool and school-aged children), Germany(15% in women of reproductive age), the United Kingdom (31% of the elderly)and New Zealand (12% of the elderly). The prevalence was lower in the UnitedStates (0–3% in preschool and school-aged children, adults and the elderly) andin Costa Rica (5.3% in lactating women). In smaller studies, a high proporti<strong>on</strong>of low plasma vitamin B 12 c<strong>on</strong>centrati<strong>on</strong>s were found in Kenya (40% in schoolagedchildren), Zimbabwe (24% of the elderly), Israel (21% in adults), and India(46% in adults), while in other countries such as Botswana (preschool-agedchildren), Thailand (school-aged children) and Japan (adults),
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDE4.3.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>Moderate to severe vitamin B 12 deficiency results in megaloblastic anaemia andthe demyelinati<strong>on</strong> of the central nervous system, and in turn, various neurologicaldisorders. The latter are variably reversible after correcti<strong>on</strong> of the deficiency(154). When serum vitamin B 12 c<strong>on</strong>centrati<strong>on</strong>s fall below 150 pmol/l, abnormalitiesin the functi<strong>on</strong> of some enzymes may occur <strong>with</strong> the risk, at lower c<strong>on</strong>centrati<strong>on</strong>,of potentially irreversible poor memory and cognitive functi<strong>on</strong>,impaired nerve c<strong>on</strong>ducti<strong>on</strong> and megaloblastic anaemia in individuals of all ages.In a peri-urban area of Guatemala City, for example, schoolchildren <strong>with</strong> lowplasma vitamin B 12 performed less well <strong>on</strong> tests of percepti<strong>on</strong> and memory, wereless accurate in a reas<strong>on</strong>ing (oddity) task, and had poorer academic performanceand adaptability (155). Infants fed <strong>with</strong> breast milk from vitamin B 12 -deficient mothers exhibited a failure to thrive, poor brain development and, insome cases, mental retardati<strong>on</strong> (156).Several studies, mainly from industrialized nati<strong>on</strong>s, have dem<strong>on</strong>strated thebenefits of vitamin B 12 supplementati<strong>on</strong> in susceptible populati<strong>on</strong> groups. Forexample, vitamin B 12 supplementati<strong>on</strong> of deficient infants born to strictly vegetarianmothers reduced the incidence of anaemia and tremors, and improvedtheir general development (156). Am<strong>on</strong>g the elderly, vitamin B 12 supplementati<strong>on</strong>produced improved symptoms in those <strong>with</strong> clinical signs of deficiency(157). To date, few vitamin B 12 interventi<strong>on</strong> trials have been carried out indeveloping countries. A recent supplementati<strong>on</strong> programme involving Kenyanschoolchildren has, however, reported significant reducti<strong>on</strong>s in the prevalenceof vitamin B 12 deficiency in those receiving supplements of meat or milk compared<strong>with</strong> placebo or energy-supplemented groups (152).4.4 Other B vitamins (thiamine, riboflavin, niacin andvitamin B 6 )As the <strong>food</strong> sources of the various B-complex vitamins are similar, it is not surprisingthat diets inadequate in <strong>on</strong>e B vitamin are more than likely to be deficientin the others. These water-soluble vitamins are readily destroyed duringcooking in water and by heat (although niacin is stable to heat). More significantly,the milling and degerming of cereal grains removes almost all of thethiamine (vitamin B 1 ), riboflavin (vitamin B 2 ) and niacin (vitamin B 3 ), which isthe reas<strong>on</strong> why restorati<strong>on</strong> of these particular nutrients to wheat and corn flourhas been widely practised for the last 60 years. This strategy has certainly c<strong>on</strong>tributedto the virtual eliminati<strong>on</strong> of vitamin B deficiencies and their associateddiseases (i.e. beriberi and pellagra) in the industrialized countries.Historically, little attenti<strong>on</strong> has been paid to the assessment of thiamine,riboflavin, niacin and vitamin B 6 status. One of the reas<strong>on</strong>s why these B-complex67
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSvitamins have been neglected in the past is the lack of reliable informati<strong>on</strong> aboutthe c<strong>on</strong>sequences of marginal or subclinical deficiencies (see Table 1.2).However, evidence is mounting that vitamin B deficiencies are highly prevalentin many developing countries, in particular where diets are low in animal products,fruits and vegetables, and where cereals are milled prior to c<strong>on</strong>sumpti<strong>on</strong>.Pregnant and lactating women, infants and children are at the highest risk ofdeficiency. Because the mother’s intake and body stores of these vitamins affectthe amount she secretes in breast milk, appropriate fortificati<strong>on</strong> can provide her<strong>with</strong> a steady supply during lactati<strong>on</strong> and thereby improve the vitamin B statusof her infants and young children.4.4.1 ThiamineThiamine (vitamin B 1 ) is a cofactor for several key enzymes involved in carbohydratemetabolism and is also directly involved in neural functi<strong>on</strong>. It is likelythat thiamine deficiency, in its subclinical form, is a significant public healthproblem in many parts of the world. Severe deficiency causes beriberi, a diseasethat was <strong>on</strong>ce comm<strong>on</strong>place am<strong>on</strong>g populati<strong>on</strong>s <strong>with</strong> a high carbohydrate intake,especially in the form of white rice. As menti<strong>on</strong>ed above, beriberi has beenlargely eradicated in most industrialized countries, but the disease still occurs insome Asian countries where rice is the staple <strong>food</strong>. In additi<strong>on</strong>, outbreaks ofberiberi are regularly reported in regi<strong>on</strong>s suffering social and ec<strong>on</strong>omic stressbrought about by war, famine and other emergency situati<strong>on</strong>s.4.4.1.1 Prevalence of deficiencyThe most widely used biochemical indicators of thiamine status are urinary thiamineexcreti<strong>on</strong> (UTE), erythrocyte thiamine transketolase activity (ETKA)and the thiamine pyrophosphate effect (TPPE), which is increased in thiaminedeficiency (see Table 4.5). UTE provides informati<strong>on</strong> about the adequacy ofdietary intakes of thiamine, but not about the degree of depleti<strong>on</strong> of tissuereserves. Nor is it a very sensitive indicator in cases of subclinical deficiency.Both ETKA and TPPE reflect tissue reserves of thiamine and provide a directfuncti<strong>on</strong>al evaluati<strong>on</strong> at the cellular level. ETKA is generally regarded as the bestsingle test of thiamine status, despite some reports of poor correlati<strong>on</strong>s betweenthis and other measures of thiamine status. Ideally, ETKA should be used incombinati<strong>on</strong> <strong>with</strong> TPPE in order to c<strong>on</strong>firm a diagnosis of thiamine deficiency.In lactating women, the c<strong>on</strong>centrati<strong>on</strong> of thiamine in breast milk can be used asan indicator of thiamine deficiency.Although the lack of reliable biochemical data means that it is not known justhow widespread a problem subclinical thiamine deficiency is, thiamine levels inbreast milk coupled <strong>with</strong> infant mortality rates can provide valuable informati<strong>on</strong><strong>on</strong> the likelihood of the existence of thiamine deficiency in a community. These68
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDETABLE 4.5Indicators for assessing thiamine (vitamin B 1 ) status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> group Cut-off to define CommentsdeficiencyMild SevereThiamine excreti<strong>on</strong> Urine 1–3 years
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 4.6Proposed criteria for assessing the public health severity of thiaminedeficiencyIndicatorSeverity of public health problem (% of populati<strong>on</strong>below the cut-off value defining deficiency, unlessotherwise stated)Mild Moderate SevereClinical signs (clinical cases) 25% 5–19 20–49 ≥50Urinary thiamine (per g creatinine) 5–19 20–49 ≥50Breast milk thiamine
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEthiaminase which is naturally present in some raw fish (166,167) and sometimesas a bacterial <strong>food</strong> c<strong>on</strong>taminant (168). Anti-thiamine compounds may also befound in tea, ferns and betel nuts (169). Chr<strong>on</strong>ic alcohol abuse and genetic disordersare also risk factors for thiamine deficiency (see Table 1.2).4.4.1.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>There are two distinct forms of severe thiamine deficiency: an oedematous formknown as wet beriberi and a n<strong>on</strong>-oedematous neurological form known as dryberiberi. The wet form is associated <strong>with</strong> potentially fatal heart failure, whereasthe dry form tends to be chr<strong>on</strong>ic and results in peripheral neuropathy. Manycases of thiamine deficiency present <strong>with</strong> a mixture of symptoms and thus areproperly termed “thiamine deficiency <strong>with</strong> cardiopathy and peripheral neuropathy”(158). Thiamine deficiency in infants is rarely seen today, and is largelyc<strong>on</strong>fined to infants who are breastfed by thiamine-deficient mothers. In suchcases, it is almost always an acute disease, involving oedema and cardiac failure<strong>with</strong> a high fatality rate.The Wernicke–Korsakov syndrome is induced by thiamine deficiency andusually manifests as various neurological disorders that are typically associated<strong>with</strong> impaired cognitive functi<strong>on</strong>. It is <strong>on</strong>ly observed in chr<strong>on</strong>ic alcoholics or inthose <strong>with</strong> a genetic abnormality in transketolase, a thiamine-dependent enzyme.Several studies have indicated that supplementati<strong>on</strong> can reverse the symptomsof thiamine deficiency. During an outbreak of beriberi in The Gambia, forexample, the affected groups resp<strong>on</strong>ded well to thiamine supplementati<strong>on</strong> (163).4.4.2 RiboflavinRiboflavin (vitamin B 2 ) is a precursor of various nucleotides, most notably flavinm<strong>on</strong><strong>on</strong>ucleotide (FMN) and flavin adenine dinucleotide (FAD), which act ascoenzymes in various metabolic pathways and in energy producti<strong>on</strong>. Riboflavindeficiency rarely occurs in isolati<strong>on</strong>, and is frequently associated <strong>with</strong> deficienciesin <strong>on</strong>e or more of the other B-complex vitamins.4.4.2.1 Prevalence of deficiencyThe urinary excreti<strong>on</strong> of riboflavin, which is reduced in case of deficiency, hasbeen used in several studies to assess riboflavin status. Urinary riboflavin reflectsrecent intake of the vitamin, but it is not a particularly good indicator of bodystores (Table 4.7). A more useful functi<strong>on</strong>al test in this respect is the erythrocyteglutathi<strong>on</strong>e reductase activity coefficient (EGRAC) (170). Erythrocyteflavin nucleotides (FMN + FAD) c<strong>on</strong>centrati<strong>on</strong> is, however, probably the bestmeasure of riboflavin status: not <strong>on</strong>ly is this less susceptible to short-term fluctuati<strong>on</strong>s,but it is also more stable than EGRAC values (171).71
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 4.7Indicators for assessing riboflavin (vitamin B2) status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> group Cut-off to define deficiency CommentsMild SevereFlavin excreti<strong>on</strong> Urine Applies to all
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEIn the few studies in which riboflavin status has been assessed at the populati<strong>on</strong>level, the prevalence of deficiency is alarmingly high (172). Abnormalriboflavin-dependent enzyme functi<strong>on</strong> has been reported in almost all pregnantwomen in The Gambia (173); in 50% of elderly and 77% of lactating women inGuatemala (174); and in 87% of night-blind women in rural Nepal (171). Furthermore,in a survey in China, urinary riboflavin was low in more than 90% ofadults (175).4.4.2.2 Risk factors for deficiencyThe main dietary sources of riboflavin are meat and dairy products; <strong>on</strong>ly smallamounts are found in grains and seeds. Leafy green vegetables are also a fairlygood source of riboflavin and in developing countries tend to be the main sourceof the vitamin. Deficiency is thus likely to be more prevalent am<strong>on</strong>g those whoseintake of animal source <strong>food</strong>s is low. In comm<strong>on</strong> <strong>with</strong> several of the otherB-complex vitamins, chr<strong>on</strong>ic alcoholism is also a risk factor.4.4.2.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>Symptoms of riboflavin deficiency are n<strong>on</strong>-specific. Early symptoms mayinclude weakness, fatigue, mouth pain, burning eyes and itching. More advanceddeficiency is characterized by dermatitis <strong>with</strong> cheilosis and angular stomatitis,brain dysfuncti<strong>on</strong> and microcytic anaemia (Table 1.2). Riboflavin deficiencyalso reduces the absorpti<strong>on</strong> and utilizati<strong>on</strong> of ir<strong>on</strong> for haemoglobin synthesis. Itis possible that riboflavin deficiency is a c<strong>on</strong>tributory factor in the high prevalenceof anaemia worldwide (see secti<strong>on</strong> 3.1.1), a suggesti<strong>on</strong> which is supportedby reports from The Gambia and Guatemala that riboflavin supplementati<strong>on</strong>improved the haemoglobin resp<strong>on</strong>se to ir<strong>on</strong> supplementati<strong>on</strong> in anaemic subjects(176,177). Almost nothing is known about the effects of milder deficiency,although depleti<strong>on</strong> studies c<strong>on</strong>ducted in the United States found evidence ofelectroencephalogram abnormalities.4.4.3 NiacinNiacin (nicotinic acid or vitamin B 3 ), as a functi<strong>on</strong>al group of the coenzymes,nicotinamide adenine dinucleotide (NAD) and its phosphate (NADP), is essentialfor oxidative processes. Deficiency results in pellagra and is associated <strong>with</strong>a heavily cereal-based diet that is low in bioavailable niacin, tryptophan (anamino acid) and other micr<strong>on</strong>utrients needed for the synthesis of niacin andtryptophan. Niacin is unique am<strong>on</strong>g the vitamins in that at least part of thebody’s requirement for it can be met through synthesis from an amino acid(tryptophan): the c<strong>on</strong>versi<strong>on</strong> of 60 mg tryptophan (via a niacin derivative) produces1 mg of niacin.73
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS4.4.3.1 Prevalence of deficiencyThere are no direct indicators of niacin status (Table 4.8). Assessment is thereforebased <strong>on</strong> the measurement of <strong>on</strong>e or preferably more urinary metabolitesof niacin, such as N’-methyl-nicotinamide (NMN) (which reflects recent dietaryintake) or the ratio of 2-pyrid<strong>on</strong>e:NMN. Provisi<strong>on</strong>al criteria proposed by WHOfor defining the severity of the public health problem based <strong>on</strong> these biomarkersare listed in Table 4.9.At present, evaluati<strong>on</strong> of the prevalence of niacin deficiency is almost entirelybased <strong>on</strong> occurrence of clinical signs of deficiency, i.e. pellagra. There is verylittle biochemical informati<strong>on</strong> <strong>on</strong> niacin status, and thus <strong>on</strong> the prevalence ofsubclinical deficiency, from developing countries.Pellagra was widespread in parts of southern Europe and in the United Statesduring the 19th and early 20th centuries, but fortificati<strong>on</strong> of cereal grain productshas since all but eradicated the c<strong>on</strong>diti<strong>on</strong> from industrialized countries. Itis, however, still comm<strong>on</strong> in India, and in parts of Africa and China, especiallywhere populati<strong>on</strong>s are dependent <strong>on</strong> maize-based diets. More recently, pellagrahas been reported in areas where diets are largely sorghum-based, and wherethere is a dependence <strong>on</strong> polished rice. The prevalence of pellagra is also higham<strong>on</strong>g displaced populati<strong>on</strong>s living in refugee camps based in south and easternparts of Africa (178). For example, up to 6.4% of Mozambican refugees basedin Malawi were affected by an outbreak of pellagra (179).4.4.3.2 Risk factors for deficiencyNiacin is widely distributed in plant and animal <strong>food</strong>s. The main sources arebaker’s yeast, animal and dairy products, cereals, legumes and leafy greenvegetables. Niacin depleti<strong>on</strong> is a risk where diets rely heavily <strong>on</strong> refined grainsor grain products and have little variety. Severe deficiency, pellagra, is predominantlyfound in people who c<strong>on</strong>sume diets that are deficient in bioavailableniacin and low in tryptophan, such as maize- or sorghum-based diets.In maize, niacin is largely present in a bound form, <strong>on</strong>ly 30% of whichbioavailable. However, the bioavailability of this bound form of niacin can beimproved by hydrolysis <strong>with</strong> a mild alkali. The soaking of maize in lime water,as is traditi<strong>on</strong>ally d<strong>on</strong>e in the preparati<strong>on</strong> of tortillas in some Latin Americancountries, releases niacin from niacytin, and thus increases the amount of niacinthat can be absorbed. Bound niacin can be also be released by heat: the roastingof coffee beans, for instance, increases the bioavailability of the nicotinic acidc<strong>on</strong>tent from 20 to 500 mg/kg (167). These practices possibly account, at leastin part, for the absence of pellagra in Latin America. The regular c<strong>on</strong>sumpti<strong>on</strong>of milk and rice can also help prevent pellagra; although they are low in niacin,milk and rice are rich in tryptophan.74
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDETABLE 4.8Indicators for assessing niacin (nicotinic acid) status at the populati<strong>on</strong> level aIndicator Sample Populati<strong>on</strong> group Cut-off to define deficiency CommentsMild SevereN′-methyl-nicotinamide Urine Adults
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 4.9Proposed criteria for assessing the public health severity of niacin deficiencyIndicatorSeverity of public health problem(% of populati<strong>on</strong> below thecut-off value defining deficiency)Mild Moderate SevereClinical signs (clinical cases)
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDETABLE 4.10Indicators for assessing vitamin B 6 (pyridoxine) status at the populati<strong>on</strong> level aIndicator Sample Populati<strong>on</strong> Cut-off to define deficiency CommentsgroupMild SeverePyridoxal 5′-phosphate Plasma Adults
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS4.4.4.2 Risk factors for deficiencyVitamin B 6 is widely distributed in <strong>food</strong>s, but meats, wholegrain products, vegetablesand nuts are especially good sources of the vitamin. Cooking and storagelosses range from a few percent to nearly half of the vitamin B 6 originally present.Plants generally c<strong>on</strong>tain pyridoxine (PN), the most stable form, while animalproducts c<strong>on</strong>tain the less stable pyridoxal (PL) and the functi<strong>on</strong>al PLP form.In comm<strong>on</strong> <strong>with</strong> several of the other B vitamins, low intakes of animal productsand a high c<strong>on</strong>sumpti<strong>on</strong> of refined cereals are the main risk factors for vitaminB 6 deficiency. Similarly, chr<strong>on</strong>ic alcoholism is an additi<strong>on</strong>al risk factor fordeficiency.4.4.4.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>Symptoms of severe vitamin B 6 deficiency are n<strong>on</strong>-specific (Table 1.2) andinclude neurological disorders (i.e. epileptic c<strong>on</strong>vulsi<strong>on</strong>s), skin changes (i.e. dermatitis,glossitis, cheilosis) and possibly anaemia. Vitamin B 6 deficiency is a riskfactor for elevated plasma homocysteine (182). In trials, vitamin B 6 supplementsincreased secreti<strong>on</strong> of the vitamin in the breast milk of lactating women (183).4.5 Vitamin CVitamin C is a redox system comprised of ascorbic acid and dehydroascorbicacid, and as such acts as an electr<strong>on</strong> d<strong>on</strong>or. Its main metabolic functi<strong>on</strong> is themaintenance of collagen formati<strong>on</strong>. It is also an important antioxidant. Althoughsevere vitamin C deficiency (scurvy) is now relatively rare, the prevalence ofmilder or marginal deficiency is probably quite high.4.5.1 Prevalence of deficiencyC<strong>on</strong>centrati<strong>on</strong>s of ascorbic acid in blood plasma or serum reflect recent intakesof vitamin C, and in this respect, are more reliable indicators of vitamin C statusthan ascorbic acid c<strong>on</strong>centrati<strong>on</strong>s in erythrocytes (Table 4.11). White blood cell(leukocyte) ascorbic acid c<strong>on</strong>centrati<strong>on</strong>s are more closely related to tissue storesand probably provide the most sensitive indicator of vitamin C status, but beingtechnically more difficult to measure, are impractical for routine and large-scalepopulati<strong>on</strong> surveys. Criteria for defining the public health significance of vitaminC deficiency, as proposed by WHO, are given in Table 4.12.Despite its near eradicati<strong>on</strong>, severe vitamin C deficiency (scurvy) still occursperiodically in displaced populati<strong>on</strong>s maintained for l<strong>on</strong>g periods of time (i.e.3–6 m<strong>on</strong>ths) <strong>on</strong> <strong>food</strong> aid and <strong>with</strong>out access to fresh fruit and vegetables (184).Outbreaks have been repeatedly reported from refugee camps in the Horn ofAfrica (i.e. Ethiopia, Kenya, Somalia, Sudan) and Nepal. In the mid-1980s, theprevalence of scurvy in refugee camps in north-west Somalia varied between78
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDETABLE 4.11Indicators for assessing vitamin C status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> group Cut-off to define deficiency CommentsMild SevereAscorbic Serum/plasma Applies to all
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 4.12Proposed criteria for assessing the public health severity of vitamin CdeficiencyIndicatorSeverity of public health problem (% of populati<strong>on</strong>)Mild Moderate SevereClinical signs (clinical cases)
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDE4.5.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>The clinical symptoms of scurvy include follicular hyperkeratosis, haemorrhagicmanifestati<strong>on</strong>s, swollen joints, swollen bleeding gums and peripheral oedema,and even death. These symptoms appear <strong>with</strong>in 3–4 m<strong>on</strong>ths of c<strong>on</strong>suming diets<strong>with</strong> a very low vitamin C c<strong>on</strong>tent (
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS4.6.1 Prevalence of deficiencyIn infants and young children, a c<strong>on</strong>centrati<strong>on</strong> of 25-OH-D in serum belowabout 27.5 nmol/l (11 ng/ml) is indicative of a low vitamin D status (Table 4.13).An elevated serum c<strong>on</strong>centrati<strong>on</strong> of alkaline phosphatase can also indicatevitamin D deficiency; alkaline phosphatase is increased in patients <strong>with</strong> ricketsor osteomalacia but is not specific to either of these c<strong>on</strong>diti<strong>on</strong>s. In adults, thecombinati<strong>on</strong> of low plasma 25-OH-D and elevated parathyroid horm<strong>on</strong>e (PTH)is probably the most reliable indicator of vitamin D deficiency (193). In theabsence of biochemical data, the existence of rickets in infants and children, anda high fracture risk am<strong>on</strong>g the elderly populati<strong>on</strong>, would suggest that vitamin Ddeficiency might be a public health problem.Breast-fed infants who are not exposed to sunlight are unlikely to obtainenough vitamin D from breast milk bey<strong>on</strong>d the first few m<strong>on</strong>ths of life, especiallyif their mother’s stores of the vitamin are low. Vitamin D deficiency ininfants as a result of low maternal stores and/or infant exposure to sunlight(especially during winter m<strong>on</strong>ths) has been reported in countries as diverse asChina (194) and France (195). Infants and children <strong>on</strong> macrobiotic diets tendto have a high prevalence of rickets, due to the low vitamin D c<strong>on</strong>tent of maternalmilk and the absence of fortified cow’s milk in their diets (196).Children living in the far northerly latitudes, whose exposure to ultravioletlight is low especially during the winter m<strong>on</strong>ths, are at high risk for rickets (197).Vitamin D deficiency is also comm<strong>on</strong> in adults living at higher latitudes: forTABLE 4.13Indicators for assessing vitamin D status at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> Cut-off to define Commentsgroupdeficiency25-hydroxyvitamin Serum Applies to all
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEinstance, surveys carried out in China after winter in populati<strong>on</strong>s living at about41°N found that 13–48% of adults were deficient in this vitamin, <strong>with</strong> the highestprevalence occurring in older men (198). In Beijing, 45% of adolescent girlswere found to be deficient (199).4.6.2 Risk factors for deficiencyMost (about 80%) of the vitamin D in the body is produced in the skin. Thisprocess usually supplies all of the vitamin D needed by infants, children andadults. However, above and below latitudes 40°N and 40°S, the intensity of ultravioletradiati<strong>on</strong> in sunlight is not sufficient to produce adequate amounts ofvitamin D in exposed skin during the 3–4 winter m<strong>on</strong>ths. At the very high latitudes,synthesis can be inadequate for as l<strong>on</strong>g as 6 m<strong>on</strong>ths of the year. Inadequatesynthesis in winter is seen as far south as Turkey and Israel; low serumlevels of vitamin D are also highly prevalent in the winter in Delhi, India (29 o N)(200). Vitamin D synthesis in the skin will also be inadequate if the body is c<strong>on</strong>sistentlycovered by clothing, a probable factor in the high prevalence of deficiencyam<strong>on</strong>g veiled women (e.g. Kuwaiti women) and their breast-fed infantsand children (201).In the elderly, dietary requirements for vitamin D are increased because theability of the skin to synthesize this vitamin decreases <strong>with</strong> age; at age 65 years,vitamin D synthesis in the skin is about 75% slower than that in younger adults.Dark-skinned individuals synthesize less vitamin D when exposed to ultravioletlight, and are therefore more vulnerable to deficiency at low levels of exposureto ultraviolet light. In the United States, cases of rickets have been reportedam<strong>on</strong>g black breast-fed children (202), and according to the results of a recentnati<strong>on</strong>al survey, 42% of African-American women had low plasma vitamin Dc<strong>on</strong>centrati<strong>on</strong>s (56).Being naturally present in relatively few <strong>food</strong>s, dietary sources of vitamin Dusually supply <strong>on</strong>ly a small fracti<strong>on</strong> of the daily requirements for the vitamin.Salt-water fish such as herring, salm<strong>on</strong>, sardines and fish liver oil are the maindietary sources. Small quantities of vitamin D are found in other animal products(e.g. beef, butter), and if hens are fed vitamin D, eggs can provide substantialamounts of the vitamin. Because the c<strong>on</strong>sumpti<strong>on</strong> of these <strong>food</strong>s tendsto be relatively low, in industrialized countries most dietary vitamin D comesfrom fortified milk and margarine. Milk <strong>on</strong>ly provides small amounts of vitaminD unless it is fortified.Several studies have shown that the effects of poor vitamin D status are exacerbatedby low calcium intakes. This has been dem<strong>on</strong>strated in adults from India(200) and in children from Nigeria (203). The Nigerian children <strong>with</strong> nutriti<strong>on</strong>alrickets resp<strong>on</strong>ded better to calcium, <strong>with</strong> or <strong>with</strong>out vitamin D, than tovitamin D al<strong>on</strong>e (203).83
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS4.6.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>The clinical features of rickets include b<strong>on</strong>e deformities and changes in the costoch<strong>on</strong>draljoints. The lesi<strong>on</strong>s are reversible after correcti<strong>on</strong> of vitamin D deficiency.In osteomalacia, in which the loss of calcium and phosphorus from b<strong>on</strong>ecauses it to lose strength, the main symptoms are muscular weakness and b<strong>on</strong>epain, but little b<strong>on</strong>e deformity. Osteomalacia c<strong>on</strong>tributes to osteoporosis, a c<strong>on</strong>diti<strong>on</strong>in which the b<strong>on</strong>e becomes more brittle and porous due to the loss ofb<strong>on</strong>e tissue. Vitamin D supplementati<strong>on</strong> reduced seas<strong>on</strong>al loss of b<strong>on</strong>e tissue inNorth American women (204), and prevented fractures associated <strong>with</strong> osteoporosisin the elderly.In many locati<strong>on</strong>s, the additi<strong>on</strong> of vitamin D to selected <strong>food</strong>s has proved tobe a prudent public health measure. The vitamin has been added to milk inCanada and the United States since the 1920s, a policy that has been largelyresp<strong>on</strong>sible for the eliminati<strong>on</strong> of vitamin D deficiency rickets in children.However, low intakes of fortified dairy products by some elderly individuals, andby some black populati<strong>on</strong>s, are still associated <strong>with</strong> a much higher risk of vitaminD deficiency am<strong>on</strong>g these groups.4.7 CalciumCalcium is the most abundant mineral in the body. Most (>99%) of the body’s1 000–1 200 g of calcium is located in the skelet<strong>on</strong> where it exists as hydroxyapatite.In additi<strong>on</strong> to its role in maintaining the rigidity and strength of the skelet<strong>on</strong>,calcium is involved in a large number of metabolic processes, includingblood clotting, cell adhesi<strong>on</strong>, muscle c<strong>on</strong>tracti<strong>on</strong>, horm<strong>on</strong>e and neurotransmitterrelease, glycogen metabolism, and cell proliferati<strong>on</strong> and differentiati<strong>on</strong>.Osteoporosis, a disease characterized by reduced b<strong>on</strong>e mass and thusincreased skeletal fragility and susceptibility to fractures, is the most significantc<strong>on</strong>sequence of a low calcium status. Although an adequacy of calcium is importantduring the whole life span, it is especially important during childhood andadolescence (as these are periods of rapid skeletal growth), and for postmenopausalwomen and the elderly whose rate of b<strong>on</strong>e loss is high.4.7.1 Prevalence of deficiencyUnfortunately there are no practical populati<strong>on</strong> level indicators of calcium status(Table 4.14). Serum calcium, for example, is regulated by a complex homeostaticmechanism, which makes it an unreliable indicator of calcium status. Forthis reas<strong>on</strong>, in most countries the prevalence of deficiency is not known. In theabsence of reliable biochemical indicators, the best indicati<strong>on</strong> of calcium adequacyat present, especially for developing countries, is probably provided bycomparing dietary intakes <strong>with</strong> recommended nutrient intakes (RNIs), despite84
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDETABLE 4.14Indicators for assessing calcium at the populati<strong>on</strong> levelIndicator Sample Populati<strong>on</strong> Cut-off to define CommentsgroupdeficiencyCalcium Serum Applies to all No universally Tightly homeostaticallypopulati<strong>on</strong> agreed cut-offs regulated and therefore doesgroups at this time not reflect calcium status.Calcium Dietary Applies to all No universally Probably the best indicator ofintake populati<strong>on</strong> agreed cut-offs calcium adequacy.groups at this timeaAt present there are no good biochemical measures for assessing calcium status.Sources: references (93,193).the variability and uncertainty in the currently recommended intakes for calcium(93,193). On the basis of the fact that intakes of dairy products are low, it isthus highly likely that low or very low calcium intakes are very comm<strong>on</strong> in developingcountries.Measurements of b<strong>on</strong>e mineral density (BMD) and b<strong>on</strong>e mineral c<strong>on</strong>tent(BMC) have provided an alternative means of assessing the likely extent ofcalcium deficiency in some countries. In the United States, for example, it hasbeen estimated that 5–6 milli<strong>on</strong> older women and 1–2 milli<strong>on</strong> older men haveosteoporosis. Other approaches include measuring markers of b<strong>on</strong>e resorpti<strong>on</strong>in urine or plasma, which tend to be higher in calcium deficient individuals.Such methods are, however, relatively expensive. All of the above measures areaffected by, am<strong>on</strong>g many other factors, vitamin D status, level of physical activityand horm<strong>on</strong>e levels, which further complicates the assessment of calciumadequacy at the populati<strong>on</strong> level.4.7.2 Risk factors for deficiencyIntakes of calcium will almost certainly fall below the recommended levels wheredairy product intake is low. Dairy products supply 50–80% of dietary calciumin most industrialized countries, while <strong>food</strong>s of plant origin supply about 25%.The calcium c<strong>on</strong>tent of, and c<strong>on</strong>tributi<strong>on</strong> from, most other <strong>food</strong>s is usually relativelysmall. Calcium absorpti<strong>on</strong> efficiency is increased by a low calcium statusand by a low dietary calcium c<strong>on</strong>tent. Absorpti<strong>on</strong> is homeostatically c<strong>on</strong>trolledthrough regulati<strong>on</strong> by vitamin D. The str<strong>on</strong>gest known inhibitor of calciumabsorpti<strong>on</strong> is dietary oxalate, followed by the presence of phytates (193). Oxalateis not an important factor in most diets (although it is high in spinach, sweetpotatoes and beans) but phytates are often c<strong>on</strong>sumed in large amounts, forinstance, in legumes and wholegrain cereals.85
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS4.7.3 Health c<strong>on</strong>sequences of deficiency and benefits of fortificati<strong>on</strong>The numerous metabolic roles of calcium are sustained even when intakes arelow, because calcium is <strong>with</strong>drawn from the b<strong>on</strong>e should homeostatic mechanismsfail to maintain an adequate calcium status in the extracellular fluid. Thusinadequate calcium intakes lead to decreased b<strong>on</strong>e mineralizati<strong>on</strong> and subsequentlyan increased risk for osteoporosis in adults (Table 1.2).In healthy individuals, b<strong>on</strong>e mineral density increases until about 30 years ofage, and thereafter begins to decline. Low intakes during childhood and adolescencecan reduce peak b<strong>on</strong>e density and thus increase the risk of osteoporosisin adulthood. The age of <strong>on</strong>set and severity of osteoporosis depends not <strong>on</strong>ly<strong>on</strong> the durati<strong>on</strong> of inadequate calcium intakes, but also <strong>on</strong> a number of otherfactors, such as estrogen levels, vitamin D status and level of physical activity.Although rickets is usually associated <strong>with</strong> vitamin D deficiency (see secti<strong>on</strong>4.6), rickets has been observed in vitamin D-replete infants who also had lowcalcium intakes (203). In Chinese children aged 5 years from China, H<strong>on</strong>g K<strong>on</strong>gSpecial Administrative Regi<strong>on</strong> (H<strong>on</strong>g K<strong>on</strong>g SAR), intakes of
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDETABLE 4.15Indicators for assessing selenium status at the populati<strong>on</strong> level aIndicator Sample Populati<strong>on</strong> group Cut-off to define deficiency CommentsSelenium Plasma, urine Applies to all 0.8–1.1 µmol/l Might reflect recent intake in low selenium envir<strong>on</strong>ments butpopulati<strong>on</strong> levels depend <strong>on</strong> the chemical form of the ingested selenium.groups Not appropriate for use in populati<strong>on</strong> surveys as technicallydifficult to measure.Selenium Erythrocytes Applies to all No universally agreed Reflects stores but not appropriate for use in populati<strong>on</strong> surveys(RBC) populati<strong>on</strong> cut-offs at this time as technically difficult to measure.groupsSelenium Hair, nails Applies to all No universally agreed Correlati<strong>on</strong>s do exist between dietary intake and hair and nailpopulati<strong>on</strong> cut-offs at this time c<strong>on</strong>centrati<strong>on</strong>s.groups C<strong>on</strong>centrati<strong>on</strong>s are affected by several factors such asfrequency of hair washing (shampoos are high in selenium)and hair colour.RBC, red blood cell.aSelenium status is probably best assessed by means of a combinati<strong>on</strong> of indicators.Sources: references (93,208).87
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSthere are no suitable biochemical indicators of selenium status that are appropriatefor use in populati<strong>on</strong> surveys. Informati<strong>on</strong> regarding the prevalence ofselenium deficiency is thus largely based <strong>on</strong> clinical observati<strong>on</strong>s and limited tothe more severe forms, i.e. Keshan or Kaschin-Beck disease.Selenium deficiency is endemic in some regi<strong>on</strong>s of China (207), whereKeshan disease was first described, and also in parts of Japan, Korea,Scandinavia and Siberia. Endemic deficiency tends to occur in regi<strong>on</strong>s characterizedby low soil selenium. For example, the distributi<strong>on</strong> of Keshan diseaseand Kaschin-Beck disease in China reflects the distributi<strong>on</strong> of soils from whichselenium is poorly available to rice, maize, wheat and pasture grasses. Fortificati<strong>on</strong>of salt and/or fertilizers <strong>with</strong> selenium is crucial in these parts of the world.4.8.2 Risk factors for deficiencyUsual diets in most countries satisfy selenium requirements. As indicated in theprevious secti<strong>on</strong>, deficiency occurs <strong>on</strong>ly where the soil, and c<strong>on</strong>sequently the<strong>food</strong>s produced <strong>on</strong> those soils, is low in available selenium. Worldwide, the seleniumc<strong>on</strong>tent of animal products and that of cereals and plants, varies widely(at least 10-fold) depending <strong>on</strong> soil selenium c<strong>on</strong>tent (209). The seleniumc<strong>on</strong>tent of <strong>food</strong>s of plant origin ranges from less than 0.1µg/g to more than0.8 µg/g, while the amount in animal products ranges from 0.1 to 1.5µg/g (210).Where animal feeds are enriched <strong>with</strong> selenium, such as in the United States,the selenium c<strong>on</strong>tent of animal products may be much higher. C<strong>on</strong>centrati<strong>on</strong>sof less than 10 ng/g in the case of grain and less than 3 ng/g in the case of watersolublesoil selenium have been proposed as indexes to define selenium-deficientareas (93).In industrialized countries, meat provides about half of the dietary selenium.It is also a good source in areas of low soil selenium because animals absorbmore of this nutrient when their intake is low. A low intake of animal source<strong>food</strong>s is thus likely to increase the risk of selenium deficiency. It is generallyassumed that the bioavailability of selenium from the diet is high.4.8.3 Health c<strong>on</strong>sequences of deficiency and benefits of interventi<strong>on</strong>Keshan disease is a cardiomyopathy associated <strong>with</strong> a low selenium intake andlow levels of selenium in blood and hair. Reports of its occurrence across a widez<strong>on</strong>e of mainland China first appeared in the mainstream scientific literature inthe 1930s. It has since also been observed in some areas of the southern Siberia.Symptoms include cardiac insufficiency and arrhythmias, c<strong>on</strong>gestive heartfailure and heart enlargement (211), which are resp<strong>on</strong>sive to supplementati<strong>on</strong><strong>with</strong> sodium selenite. Because some features of Keshan disease cannot beexplained by selenium deficiency al<strong>on</strong>e, other c<strong>on</strong>tributing factors have beensuggested, in particular, infecti<strong>on</strong> <strong>with</strong> the cocksackie virus (212).88
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEThe selenium deficiency syndrome known as Kaschin-Beck or Urov diseaseis found in parts of China and Siberia, and in Japan and Korea. This is a diseaseof cartilage tissue that occurs in pre-adolescent and adolescent children, causingosteoarthropathy, joint problems and growth stunting. Like Keshan disease,additi<strong>on</strong>al causal factors have been proposed to account for the etiology ofKeschin-Beck disease, including exposure to mycotoxins from Fusarium mould(213), mineral imbalances and iodine deficiency (214).Low intakes of selenium have been linked to a reduced c<strong>on</strong>versi<strong>on</strong> of thethyroid horm<strong>on</strong>e, T 4 to T 3 . The metabolic interrelati<strong>on</strong>s between selenium andiodine are such that deficiencies in <strong>on</strong>e can sometimes exacerbate problems <strong>with</strong>the other. In the Democratic Republic of C<strong>on</strong>go, for instance, combined seleniumand iodine deficiencies were shown to c<strong>on</strong>tribute to endemic myxoedematouscretinism. Administrati<strong>on</strong> of selenium al<strong>on</strong>e appeared to aggravate thisdisease; by restoring selenium-dependent deiodinase activity, the synthesis anduse of thyroxine (T 4 ) and iodine is increased, thereby exacerbating the iodinedeficiency (215). Low selenium intakes have also been associated by someresearchers <strong>with</strong> an increased incidence of cancer, in particular, oesophagealcancer and also <strong>with</strong> cardiovascular disease (216).In areas of endemic selenium deficiency, fortificati<strong>on</strong> <strong>with</strong> selenium has beenshown to rapidly increase plasma glutathi<strong>on</strong>e peroxidase levels and urinary selenium.For example, when selenium was added to fertilizers in Finland in 1984,plasma selenium levels doubled by 1991 and glutathi<strong>on</strong>e peroxidase activity wasnormalized (217). In additi<strong>on</strong>, according to the results of large-scale survey(over 1 milli<strong>on</strong> people) selenium fortificati<strong>on</strong> of table salt has significantlyreduced the prevalence of Keshan disease in China (218).4.9 FluorideUnlike the other micr<strong>on</strong>utrients c<strong>on</strong>sidered in these guidelines, fluoride is notgenerally c<strong>on</strong>sidered to be an essential nutrient according to the strict definiti<strong>on</strong>of the term (see Chapter 2: secti<strong>on</strong> 2.1.1). Nevertheless, fluoride is undoubtedlyprotective against tooth decay.4.9.1 Prevalence of dental cariesThere are no universally agreed methods for assessing fluoride status and nogenerally accepted criteria <strong>with</strong> which to define deficiency. However, c<strong>on</strong>centrati<strong>on</strong>sin urine have sometimes been used as an indicator of fluoride status(Table 4.16).The prevalence of dental caries is 40–60% lower in those areas of the UnitedStates where water is fluoridated compared <strong>with</strong> those where it is not. However,the increased use of fluoridated toothpaste and supplements by infants and89
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 4.16Indicators for assessing fluoride status at the populati<strong>on</strong> level aIndicator Sample Populati<strong>on</strong> Cut-off to define CommentsgroupdeficiencyFluoride Urine Applies to all
4. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEfluorosis, <strong>with</strong> symptoms that include b<strong>on</strong>e pain, and in more severe cases,muscle calcificati<strong>on</strong> and crippling. Mild skeletal fluorosis <strong>on</strong>ly occurs at fluorideintakes that are in excess of 10 mg/day for more than 10 years. Symptoms ofskeletal fluorosis are rarely seen in communities where the fluoride c<strong>on</strong>tent ofwater supplies is below 20 ppm (20 mg/l).4.10 Multiple micr<strong>on</strong>utrient deficiencies4.10.1 Prevalence and risk factorsBased <strong>on</strong> what is known about the prevalence of deficiencies in individualmicr<strong>on</strong>utrients, it is probable that multiple micr<strong>on</strong>utrient deficiencies arecomm<strong>on</strong> in several parts of the world and in certain populati<strong>on</strong> groups.Micr<strong>on</strong>utrient deficiencies are more likely to coexist in individuals who c<strong>on</strong>sumediets that are poor in nutriti<strong>on</strong>al quality, or who have higher nutrient requirementsdue to high growth rates and/or the presence of bacterial infecti<strong>on</strong>s orparasites. In particular, a diet that is low in animal source <strong>food</strong>s typically resultsin low intakes of bioavailable ir<strong>on</strong> and zinc, calcium, retinol (pre-formed vitaminA), vitamin B 2 (riboflavin), vitamin B 6 and vitamin B 12 . Often, poor quality dietsalso lack fresh fruits and vegetables, which means that intakes of vitamin C(ascorbic acid), β-carotene (provitamin A) and folate will also be inadequate.The milling of cereals removes several nutrients, notably, ir<strong>on</strong> and zinc, variousB vitamins (i.e. thiamine, riboflavin, niacin) and folate. Individuals who relyheavily <strong>on</strong> refined cereals are thus at increased risk of deficiency of all of thesemicr<strong>on</strong>utrients. The breast milk of undernourished lactating women c<strong>on</strong>suminga limited range of <strong>food</strong>s and <strong>with</strong> multiple micr<strong>on</strong>utrient deficiencies, is mostlikely to be low in c<strong>on</strong>centrati<strong>on</strong>s of vitamin A (retinol), the B vitamins, iodineand selenium. If the micr<strong>on</strong>utrient c<strong>on</strong>tent of breast milk is inadequate foroptimal infant development, maternal supplementati<strong>on</strong> may be required untiladequate fortificati<strong>on</strong> programmes can be launched.4.10.2 Health c<strong>on</strong>sequences and benefits of interventi<strong>on</strong>As several previous subsecti<strong>on</strong>s have indicated, a deficiency in <strong>on</strong>e micr<strong>on</strong>utrientcan impair the utilizati<strong>on</strong> of another. C<strong>on</strong>versely, improving an individual’sstatus in <strong>on</strong>e micr<strong>on</strong>utrient, or even several micr<strong>on</strong>utrients simultaneously in thecase of multiple deficiencies, can have wider benefits. For example, ir<strong>on</strong> deficiencymay cause vitamin A to be trapped in the liver; several studies have shownthat ir<strong>on</strong> supplementati<strong>on</strong> al<strong>on</strong>e can increase serum retinol c<strong>on</strong>centrati<strong>on</strong>smarkedly (85). Goitre is more resistant to improvement by iodine supplementati<strong>on</strong>in the presence of ir<strong>on</strong> deficiency, and ir<strong>on</strong> supplementati<strong>on</strong> of deficientchildren improves their rate of goitre resp<strong>on</strong>se to iodine supplements or iodinefortified salt (87). Similarly, the additi<strong>on</strong> of vitamin A to ir<strong>on</strong> supplements91
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSincreases blood haemoglobin by a substantial amount in vitamin A-depleted,anaemic populati<strong>on</strong>s (99) and can help to further increase ir<strong>on</strong> stores (223).Deficiencies of vitamin B 12 , folate, vitamin B 2 (riboflavin) and several othermicr<strong>on</strong>utrients can also c<strong>on</strong>tribute to anaemia (77). As vitamin C (from <strong>food</strong>sor added as a fortificant) improves the absorpti<strong>on</strong> of n<strong>on</strong>-haem ir<strong>on</strong> from<strong>food</strong> and many ir<strong>on</strong> fortificants, it too is frequently added as well as ir<strong>on</strong> as afortificant.In the past, interventi<strong>on</strong>s have targeted deficiencies in ir<strong>on</strong>, vitamin A andiodine, in part because these can be detected more easily and more is knownabout their adverse effects. Typically, separate programmes were developed foreach nutrient. In more recent years, it has become increasingly apparent thatthere are many reas<strong>on</strong>s why multiple micr<strong>on</strong>utrient fortificati<strong>on</strong> may be moreappropriate and should be c<strong>on</strong>sidered. In additi<strong>on</strong> to treating and preventingir<strong>on</strong>, vitamin A and iodine deficiencies, fortificati<strong>on</strong> affords a good opportunityto c<strong>on</strong>trol other micr<strong>on</strong>utrient deficiencies that are likely to coexist in manypopulati<strong>on</strong>s.92
PA RT I I IFortificants: physicalcharacteristics, selecti<strong>on</strong> anduse <strong>with</strong> specific <strong>food</strong> vehicles
Introducti<strong>on</strong>By providing a critical review of the fortificants that are currently available forfortificati<strong>on</strong> purposes, Part III of these guidelines is intended to assist programmemanagers in their choice of firstly, a suitable <strong>food</strong> vehicle and sec<strong>on</strong>dly,a compatible fortificant. Having established – through the applicati<strong>on</strong> of appropriatecriteria – that the nature of the public health risk posed by a micr<strong>on</strong>utrientdeficiency justifies interventi<strong>on</strong> in the form of <strong>food</strong> fortificati<strong>on</strong>, the selecti<strong>on</strong>of a suitable combinati<strong>on</strong> of <strong>food</strong> vehicle and fortificant(s), or more specifically,the chemical form of the micr<strong>on</strong>utrient(s) that will added to the chosen <strong>food</strong>vehicle, is fundamental to any <strong>food</strong> fortificati<strong>on</strong> programme. Subsequent chapters(Part IV) cover other important aspects of <strong>food</strong> fortificati<strong>on</strong> programmeplanning, including how to calculate how much fortificant to add to the chosen<strong>food</strong> vehicle in order to achieve a predetermined public health benefit (Chapter7), m<strong>on</strong>itoring and impact evaluati<strong>on</strong> (Chapters 8 and 9), marketing (Chapter10) and regulatory issues (Chapter 11).In practice, the selecti<strong>on</strong> of a <strong>food</strong> vehicle–fortificant combinati<strong>on</strong> is governedby range of factors, both technological and regulatory. Foods such as cereals,oils, dairy products, beverages and various c<strong>on</strong>diments such as salt, sauces (e.g.soy sauce) and sugar are particularly well suited to mandatory mass fortificati<strong>on</strong>.These <strong>food</strong>s share some or all of the following characteristics:• They are c<strong>on</strong>sumed by a large proporti<strong>on</strong> of the populati<strong>on</strong>, including (orespecially) the populati<strong>on</strong> groups at greatest risk of deficiency.• They are c<strong>on</strong>sumed <strong>on</strong> a regular basis, in adequate and relatively c<strong>on</strong>sistentamounts.• They can be centrally processed (central processing is preferable for anumber of reas<strong>on</strong>s, but primarily because the fewer the number of locati<strong>on</strong>swhere fortificants are added, the easier it is to implement quality c<strong>on</strong>trol measures;m<strong>on</strong>itoring and enforcement procedures are also likely to be more effective).• Allow a nutrient premix to be added relatively easily using low-cost technology,and in such a way so as to ensure an even distributi<strong>on</strong> <strong>with</strong>in batches ofthe product.95
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS• Are used relatively so<strong>on</strong> after producti<strong>on</strong> and purchase. Foods that are purchasedand used <strong>with</strong>in a short period of time of processing tend to havebetter vitamin retenti<strong>on</strong>, and fewer sensorial changes due to the need for <strong>on</strong>lya small overage 1 .The choice of fortificant compound is often a compromise between reas<strong>on</strong>ablecost, bioavailability from the diet, and the acceptance of any sensory changes.When selecting the most appropriate chemical form of a given micr<strong>on</strong>utrient,the main c<strong>on</strong>siderati<strong>on</strong>s and c<strong>on</strong>cerns are thus:• Sensory problems. Fortificants must not cause unacceptable sensory problems(e.g. colour, flavour, odour or texture) at the level of intended fortificati<strong>on</strong>,or segregate out from the <strong>food</strong> matrix, and they must be stable <strong>with</strong>in givenlimits. If additi<strong>on</strong>al packaging is needed to improve stability of the added fortificant,it is helpful if this does not add significantly to the cost of the productand make it unaffordable to the c<strong>on</strong>sumer.• Interacti<strong>on</strong>s. The likelihood or potential for interacti<strong>on</strong>s between the addedmicr<strong>on</strong>utrient and the <strong>food</strong> vehicle, and <strong>with</strong> other nutrients (either added ornaturally present), in particular any interacti<strong>on</strong>s that might interfere <strong>with</strong> themetabolic utilizati<strong>on</strong> of the fortificant, needs to be assessed and checked priorto the implementati<strong>on</strong> of a fortificati<strong>on</strong> programme.• Cost. The cost of fortificati<strong>on</strong> must not affect the affordability of the <strong>food</strong> norits competitivity <strong>with</strong> the unfortified alternative.• Bioavailability. The fortificant must be sufficiently well absorbed from the<strong>food</strong> vehicle and be able to improve the micr<strong>on</strong>utrient status of the targetpopulati<strong>on</strong>.Safety is also an important c<strong>on</strong>siderati<strong>on</strong>. The level of c<strong>on</strong>sumpti<strong>on</strong> that isrequired for fortificati<strong>on</strong> to be effective must be compatible <strong>with</strong> a healthy diet.The following two chapters c<strong>on</strong>sider the above factors in relati<strong>on</strong> to specificmicr<strong>on</strong>utrients or micr<strong>on</strong>utrient groups. Chapter 5 deals <strong>with</strong> ir<strong>on</strong>, vitamin Aand iodine; Chapter 6 covers some of the other micr<strong>on</strong>utrients (such as zinc,folate and the other B vitamins, vitamin D and calcium) for which the severityof the public health problem of deficiencies is less well known but is believed tobe significant. The discussi<strong>on</strong> is limited to those fortificants and <strong>food</strong> vehiclesthat currently are the most widely used, or that have potential for wider applicati<strong>on</strong>.Details of publicati<strong>on</strong>s and articles c<strong>on</strong>taining more in-depth informati<strong>on</strong>about the fortificati<strong>on</strong> of <strong>food</strong>s <strong>with</strong> specific nutrients are provided in theattached further reading list.1Overage is the term used to describe the extra amount of micr<strong>on</strong>utrient that is added to a <strong>food</strong>vehicle to compensate for losses during producti<strong>on</strong>, storage, distributi<strong>on</strong> and selling.96
CHAPTER 5Ir<strong>on</strong>, vitamin A and iodine5.1 Ir<strong>on</strong>5.1.1 Choice of ir<strong>on</strong> fortificantTechnically, ir<strong>on</strong> is the most challenging micr<strong>on</strong>utrient to add to <strong>food</strong>s, becausethe ir<strong>on</strong> compounds that have the best bioavailability tend to be those that interactmost str<strong>on</strong>gly <strong>with</strong> <strong>food</strong> c<strong>on</strong>stituents to produce undesirable organolepticchanges. When selecting a suitable ir<strong>on</strong> compound as a <strong>food</strong> fortificant, theoverall objective is to find the <strong>on</strong>e that has the greatest absorbability, i.e. thehighest relative bioavailability 1 (RBV) compared <strong>with</strong> ferrous sulfate, yet atthe same time does not cause unacceptable changes to the sensory properties(i.e. taste, colour, texture) of the <strong>food</strong> vehicle. Cost is usually another importantc<strong>on</strong>siderati<strong>on</strong>.A wide variety of ir<strong>on</strong> compounds are currently used as <strong>food</strong> fortificants(Table 5.1). These can be broadly divided into three categories: (224–226)—water soluble;— poorly water soluble but soluble in dilute acid;—water insoluble and poorly soluble in dilute acid.5.1.1.1 Water-soluble compoundsBeing highly soluble in gastric juices, the water-soluble ir<strong>on</strong> compounds havethe highest relative bioavailabilities of all the ir<strong>on</strong> fortificants and for this reas<strong>on</strong>are, more often than not, the preferred choice. However, these compounds arealso the most likely to have adverse effects <strong>on</strong> the organoleptic qualities of <strong>food</strong>s,in particular, <strong>on</strong> the colour and flavour. During prol<strong>on</strong>ged storage, the presenceof fortificant ir<strong>on</strong> in certain <strong>food</strong>s can cause rancidity and subsequent offflavours.Moreover, in the case of multiple fortificati<strong>on</strong>, free ir<strong>on</strong>, produced fromthe degradati<strong>on</strong> of ir<strong>on</strong> compounds present in the <strong>food</strong>, can oxidize some of thevitamins supplied in the same fortificant mixture.1Relative bioavailability is a measure which scores the absorbability of a nutrient by comparingits absorbability to that of a reference nutrient that is c<strong>on</strong>sidered as having the most efficientabsorbability.97
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 5.1Key characteristics of ir<strong>on</strong> compounds comm<strong>on</strong>ly used for <strong>food</strong> fortificati<strong>on</strong>purpose: solubility, bioavailability and costCompound Ir<strong>on</strong> c<strong>on</strong>tent Relative bioavailability a Relative cost b(%) (per mg ir<strong>on</strong>)Water solubleFerrous sulfate. 7H 2 0 20 100 1.0Ferrous sulfate, dried 33 100 1.0Ferrous gluc<strong>on</strong>ate 12 89 6.7Ferrous lactate 19 67 7.5Ferrous bisglycinate 20 >100 c 17.6Ferric amm<strong>on</strong>ium citrate 17 51 4.4Sodium ir<strong>on</strong> EDTA 13 >100 c 16.7Poorly water soluble, soluble in dilute acidFerrous fumarate 33 100 2.2Ferrous succinate 33 92 9.7Ferric saccharate 10 74 8.1Water insoluble, poorly soluble in dilute acidFerric orthophosphate 29 25–32 4.0Ferric pyrophosphate 25 21–74 4.7Elemental ir<strong>on</strong> – – –H-reduced 96 13–148 d 0.5Atomized 96 (24) 0.4CO-reduced 97 (12–32)
5. IRON, VITAMIN A AND IODINEto physically separate the ir<strong>on</strong> from the other <strong>food</strong> comp<strong>on</strong>ents, can be used forslowing down or preventing sensory changes.Ferrous sulfate is by far the most frequently used water-soluble ir<strong>on</strong> fortificant,principally because it is the cheapest. It has been widely used to fortifyflour (see secti<strong>on</strong> 5.1.5.1). However, depending <strong>on</strong> its physical characteristics,the climate and the fat c<strong>on</strong>tent of the flour to which it is added, ferrous sulfatecan cause rancidity, and therefore its suitability as a fortificant needs to be evaluatedin trials before use.5.1.1.2 Ir<strong>on</strong> compounds that are poorly soluble in water but soluble indilute acidCompounds that fall into the sec<strong>on</strong>d category of ir<strong>on</strong> fortificants (see Table 5.1)are also reas<strong>on</strong>ably well absorbed from <strong>food</strong>, as they are soluble in the gastricacids produced in the stomach of normal healthy adults and adolescents. Somec<strong>on</strong>cern has been raised about absorpti<strong>on</strong> in infants who may secrete less acidbut further research is needed in this area before any firm c<strong>on</strong>clusi<strong>on</strong>s can bedrawn. In most people, however, <strong>with</strong> the possible excepti<strong>on</strong> of individuals whosuffer from a lack of gastric acid due to medical problems, ir<strong>on</strong> absorpti<strong>on</strong> fromthese compounds is likely to be similar to that from water-soluble ir<strong>on</strong> compounds.Poorly water-soluble ir<strong>on</strong> compounds, such as ferrous fumarate, havethe advantage of causing fewer sensory problems in <strong>food</strong>s than the water-solublecompounds, and are generally next in line for c<strong>on</strong>siderati<strong>on</strong>, especially if morewater-soluble forms cause unacceptable organoleptic changes in the chosen <strong>food</strong>vehicle.Ferrous fumarate and ferric saccharate are the most comm<strong>on</strong>ly used ir<strong>on</strong>compounds in this group, and in adults are as bioavailable as ferrous sulfate.The former is frequently used to fortify infant cereals and the latter, chocolatedrink powders. Ferrous fumarate is used to fortify maize flour in Venezuela andwheat flour in Central America, where it has also been proposed as a potentialfortificant for maize masa. Ferrous fumarate can be used in an encapsulatedform to limit sensory changes.5.1.1.3 Ir<strong>on</strong> compounds that are insoluble in water and poorly soluble indilute acidRelative to ferrous sulfate, the absorpti<strong>on</strong> of ir<strong>on</strong> from water-insoluble compoundsranges from approximately 20% up to 75%. Despite their reducedabsorbability, water-insoluble ir<strong>on</strong> compounds have been widely used by the<strong>food</strong> industry as fortificants because they have far less effect <strong>on</strong> the sensoryproperties of <strong>food</strong>s (at the levels currently used) and because they are cheaperthan the more soluble compounds. However, they are generally regarded as thelast resort opti<strong>on</strong>, especially in settings where the diet of the target populati<strong>on</strong>99
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSis high in ir<strong>on</strong> absorpti<strong>on</strong> inhibitors. If it is necessary to use a water-insolubleir<strong>on</strong> fortificant, it should ideally have an absorpti<strong>on</strong> equivalent to at least 50%that of ferrous sulfate (as measured in rat or human assays), and twice as muchwould need to be added in order to compensate for the reduced absorpti<strong>on</strong>rate.Within this category of ir<strong>on</strong> fortificants, the ferric phosphate compounds –ferric orthophosphate and ferric pyrophosphate – are used to fortify rice, andsome infant cereals and chocolate-c<strong>on</strong>taining <strong>food</strong>s. They have a modest ir<strong>on</strong>bioavailability: the relative bioavailability of ferric pyrophosphate is reported tobe 21–74%, and that of ferric orthophosphate, 25–32%. However, the relativebioavailability of the ferric phosphates may change during the processing of a<strong>food</strong> (227,228).Elemental ir<strong>on</strong> powders are used in a number of countries to fortify cereals,but the bioavailabilities of the different forms of elemental ir<strong>on</strong> that are currentlyavailable (Table 5.1) are not well established (229). The solubility of elementalir<strong>on</strong> is very dependent <strong>on</strong> the size, shape and surface area of the ir<strong>on</strong> particles(characteristics which are governed by the manufacturing process 1 ), as well asthe compositi<strong>on</strong> of the meals in which it is c<strong>on</strong>sumed.According to the c<strong>on</strong>clusi<strong>on</strong>s of the Sharing United States Technology to AidImprovement of Nutriti<strong>on</strong> (SUSTAIN) Task Force, <strong>on</strong>ly electrolytic ir<strong>on</strong>powders (diameter 149 micr<strong>on</strong>s or 100 mesh) is probably too insoluble in the intestine and istherefore not generally recommended for use as a <strong>food</strong> fortificant. Furthertesting of the bioavailability of various elemental ir<strong>on</strong> powders is <strong>on</strong>going (42).5.1.2 Methods used to increase the amount of ir<strong>on</strong> absorbedfrom fortificantsThe bioavailability of ir<strong>on</strong> from fortificants is dependent not <strong>on</strong>ly <strong>on</strong> the solubilityof the fortificant as discussed above, but also <strong>on</strong> the compositi<strong>on</strong> of the1For more details, please refer to the Handbook of powder metal technologies and applicati<strong>on</strong>s (230).2At the time of writing, Glidden 131 was still available.100
5. IRON, VITAMIN A AND IODINEdiet, in particular, <strong>on</strong> the proporti<strong>on</strong> of inhibitors of ir<strong>on</strong> absorpti<strong>on</strong> in the diet,notably ir<strong>on</strong>-binding phytates and certain phenolic compounds. The additi<strong>on</strong> ofascorbic acid (vitamin C) or sodium ethylenediaminetetraacetic acid (sodiumEDTA or Na 2 EDTA) and the removal of phytates, all of which reduce the effectof the inhibitors, can be effective ways of increasing the total amount of ir<strong>on</strong>absorbed from ir<strong>on</strong>-fortified <strong>food</strong>s.5.1.2.1 Ascorbic acidThe additi<strong>on</strong> of ascorbic acid causes a substantial increase in the amount of ir<strong>on</strong>absorbed from most ir<strong>on</strong> compounds (40,224). Ascorbic acid additi<strong>on</strong> to ir<strong>on</strong>fortified<strong>food</strong>s is thus a widely adopted practice throughout the <strong>food</strong> industry,especially for processed <strong>food</strong>s. This opti<strong>on</strong> is, however, not recommended forstaples and c<strong>on</strong>diments because of stability issues (see secti<strong>on</strong> 5.1.5.1). Forexample, Chile fortifies milk powder delivered through its public health programme<strong>with</strong> both ir<strong>on</strong> and ascorbic acid (as well as some other micr<strong>on</strong>utrients)to c<strong>on</strong>trol anaemia in infants and young children.In most studies, the co-additi<strong>on</strong> of ascorbic acid and ir<strong>on</strong> in a 2 : 1 molar ratio(6 : 1 weight ratio) increased ir<strong>on</strong> absorpti<strong>on</strong> from <strong>food</strong>s 2- to 3-fold in adultsand children (224). This ratio of ascorbic acid to ir<strong>on</strong> is thus recommended formost <strong>food</strong>s; a higher ascorbic acid:ir<strong>on</strong> molar ratio (4 : 1) can be used for highphytate<strong>food</strong>s. The main problem <strong>with</strong> using ascorbic acid as a <strong>food</strong> additiveis that substantial amounts can be lost during <strong>food</strong> storage and preparati<strong>on</strong>.This means that, relative to some of the alternatives, it can be an expensiveopti<strong>on</strong>.5.1.2.2 Sodium EDTASodium EDTA is a permitted <strong>food</strong> additive in many countries, and unlike ascorbicacid, is stable during processing and storage. At low pH (i.e. in the stomach),sodium EDTA acts as a chelating agent, and as such prevents ir<strong>on</strong> from bindingto phytic acid or phenolic compounds, which would otherwise inhibit ir<strong>on</strong>absorpti<strong>on</strong> (231). Its additi<strong>on</strong> enhances the absorpti<strong>on</strong> of both <strong>food</strong> ir<strong>on</strong> andsoluble ir<strong>on</strong> fortificants (232), but not that of the relatively insoluble ir<strong>on</strong> compoundssuch as ferrous fumarate (233), ferric pyrophosphate (232) or elementalir<strong>on</strong> (234).In the case of <strong>food</strong>s fortified <strong>with</strong> soluble ir<strong>on</strong> compounds, such as ferroussulfate, the additi<strong>on</strong> of sodium EDTA in a molar ratio of Na 2 EDTA:ir<strong>on</strong> ofbetween 0.5 and 1.0 (between 3.3 : 1 and 6.6 : 1 weight ratio) is recommended.Under these circumstances ir<strong>on</strong> absorpti<strong>on</strong> is increased by up to 2–3 times(224).101
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS5.1.2.3 Dephytinize cereals and legumesThe phytic acid c<strong>on</strong>tent of cereals, pulses and legumes can be substantiallyreduced by several methods (224), some of which are particularly suitable forensuring adequate ir<strong>on</strong> absorpti<strong>on</strong> from cereal-based complementary <strong>food</strong>s orsoy-based infant formulas. However, the molar ratio of phytic acid:ir<strong>on</strong> needsto be decreased to at least 1 : 1, or even to less than 0.5 : 1, in order to achieve ameaningful increase in ir<strong>on</strong> absorpti<strong>on</strong>.Milling removes about 90% of the phytic acid from cereal grains, but theremaining 10% is still str<strong>on</strong>gly inhibitory. The acti<strong>on</strong> of phytases (enzymes) isusually necessary in order to achieve complete phytate degradati<strong>on</strong>. Naturallyoccurringcereal phytases can be activated by traditi<strong>on</strong>al processes, such assoaking, germinati<strong>on</strong> and fermentati<strong>on</strong>. At the industrial level, it is possible tocompletely degrade phytic acid in complementary <strong>food</strong> mixtures of cereals andlegumes by adding exogenous phytases or by adding whole wheat or whole ryeas a source of phytases, these being naturally high in phytases (224,235–237).Because of the risk of bacterial c<strong>on</strong>taminati<strong>on</strong>, it is better to add the phytasesunder factory c<strong>on</strong>diti<strong>on</strong>s, but as yet, this practice has not been adoptedcommercially.5.1.3 Novel ir<strong>on</strong> fortificantsIn recent years, c<strong>on</strong>siderable effort has been devoted to the development andtesting of alternative ir<strong>on</strong> fortificants, in particular, fortificants that provide betterprotecti<strong>on</strong> against ir<strong>on</strong> absorpti<strong>on</strong> inhibitors than those currently available.Am<strong>on</strong>g those at an experimental stage are sodium ir<strong>on</strong> EDTA (NaFeEDTA),ferrous bisglycinate and various encapsulated and micr<strong>on</strong>ized ir<strong>on</strong> compounds.In recent years, NaFeEDTA has been selected as the ir<strong>on</strong> compound to fortifygovernment-led soy sauce fortificati<strong>on</strong> and wheat flour fortificati<strong>on</strong> programs inChina, and fish sauce fortificati<strong>on</strong> in Vietnam.5.1.3.1 Sodium ir<strong>on</strong> EDTAIn high-phytate <strong>food</strong>s, the absorpti<strong>on</strong> of ir<strong>on</strong> from NaFeEDTA is 2–3 timesgreater than that from either ferrous sulfate or ferrous fumarate. In <strong>food</strong>s <strong>with</strong>a low phytate c<strong>on</strong>tent, however, ir<strong>on</strong> absorpti<strong>on</strong> is similar (231,232). In additi<strong>on</strong>to better absorpti<strong>on</strong> from high-phytate fortified <strong>food</strong>s, NaFeEDTA offersa number of other advantages: it does not promote lipid oxidati<strong>on</strong> in storedcereals, or the formati<strong>on</strong> of precipitates in <strong>food</strong>s that are high in free peptides,such as soy sauce and fish sauce. On the down side, it is expensive, and becauseit is slowly soluble in water, it may cause colour changes in some <strong>food</strong>s.The Joint FAO/WHO Expert Committee <strong>on</strong> Food Additives has approvedthe use of NaFeEDTA at 0.2 mg Fe/kg body weight per day (238). Nevertheless,the use of Na 2 EDTA plus ferrous sulfate (or possibly other soluble ir<strong>on</strong>102
5. IRON, VITAMIN A AND IODINEcompounds) rather than NaFeEDTA might yet prove to be the better opti<strong>on</strong>for high-phytate <strong>food</strong>s. In most settings, the choice will depend <strong>on</strong> the relativecosts of, and accessibility to, the EDTA compounds, the acceptability of sensorychanges in the <strong>food</strong>, and current legislati<strong>on</strong>.5.1.3.2 Ferrous bisglycinateFerrous bisglycinate is an ir<strong>on</strong>–amino acid chelate in which the ir<strong>on</strong> is protectedfrom the acti<strong>on</strong> of absorpti<strong>on</strong> inhibitors by being bound to the amino acid,glycine. Absorpti<strong>on</strong> from this form of ir<strong>on</strong> has been reported to be 2–3 timesbetter than that from ferrous sulfate in a high-phytate cereal and in whole maize.In c<strong>on</strong>trast, a closely-related compound, ferric trisglycinate, is not well absorbedfrom maize (239,240).Ferrous bisglycinate seems to be particularly well suited to the fortificati<strong>on</strong> ofliquid whole milk and other dairy products where use of ferrous sulfate leads torancid off-flavours. However, ferrous bisglycinate can also cause rancidity byoxidizing fats in <strong>food</strong>, which can be a problem in cereal flours and weaningcereals unless an antioxidant is added as well. Furthermore, the bisglycinate ismuch more expensive than many other ir<strong>on</strong> compounds.5.1.3.3 Encapsulated ferrous sulfate and ferrous fumarateSeveral ir<strong>on</strong> compounds are available commercially in encapsulated form,namely ferrous sulfate and ferrous fumarate, and are currently used in dry infantformulas and in infant cereals, predominantly in industrialized countries. Infuture, use of encapsulated forms of ir<strong>on</strong> compounds may extend to developingcountries, although their cost may be a problem. Encapsulati<strong>on</strong> increasescosts 3- to 5-fold, which when expressed in terms of ir<strong>on</strong> amounts, is equivalentto a 10-fold increase in cost relative to the use of dried ferrous sulfate(Table 5.1).As previously indicated, the main purpose of encapsulati<strong>on</strong> is to separate their<strong>on</strong> from the other <strong>food</strong> comp<strong>on</strong>ents, thereby mitigating sensory changes. Indouble fortified salt (i.e. salt fortified <strong>with</strong> iodine and ir<strong>on</strong>), encapsulati<strong>on</strong> ofir<strong>on</strong> has been shown to help prevent iodine losses and to slow down colourchanges.When developing encapsulated ir<strong>on</strong> fortificants, it is important to select acoating that provides an adequate balance between stability and bioavailability.Ir<strong>on</strong> compounds are usually encapsulated <strong>with</strong> hydrogenated vegetable oils, butm<strong>on</strong>o- and diglycerides, maltodextrins and ethyl cellulose, have also been used.Because of the different methods of manufacture, and because different capsulematerials and thicknesses are possible, it is imperative to c<strong>on</strong>firm bioavailability,at least in rat assays, before widespread use as a fortificant. Tests have shownthat encapsulati<strong>on</strong> of ferrous sulfate and ferrous fumarate does not alter ir<strong>on</strong>103
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSbioavailability to rats. In additi<strong>on</strong>, dual fortificati<strong>on</strong> of salt <strong>with</strong> encapsulated ir<strong>on</strong>has been found to be efficacious in humans (see secti<strong>on</strong> 1.3.2.3) (44).5.1.3.4 Micr<strong>on</strong>ized ferric pyrophosphateJust as the bioavailability of elemental ir<strong>on</strong> powders is increased by reducingtheir particle size, so too can that of insoluble ir<strong>on</strong> salts, such as ferric pyrophosphate.Micr<strong>on</strong>izing insoluble ir<strong>on</strong> salts to an extremely small submicr<strong>on</strong> particlesize cannot, however, be achieved by physical grinding, <strong>on</strong>ly by a chemicalprocess.A micr<strong>on</strong>ized form of ferric pyrophosphate (diameter, 0.5 micr<strong>on</strong>s) has beendeveloped recently for use as a <strong>food</strong> fortificant. It is available in both liquid anddried forms. In order to make it dispersible in liquids, the particles of ferricpyrophosphate are coated <strong>with</strong> emulsifiers. Relative to ordinary ferric pyrophosphate(mean particle size of around 8 micr<strong>on</strong>s), ir<strong>on</strong> absorpti<strong>on</strong> by adult humansis improved by 2–4 four times in milk products (241). Its principal advantageis that, being insoluble in water, it is unlikely to cause many sensory problems,although this remains to be tested adequately. Currently it is added to liquid milkand yoghurt products in Japan, but its more widespread use in the foreseeablefuture is prohibited by its very high cost.5.1.4 Sensory changesIn the case of ir<strong>on</strong> fortificants, the two most comm<strong>on</strong> problems are increasedrancidity due to oxidati<strong>on</strong> of unsaturated lipids and unwanted colour changes.The latter typically include a green or bluish colourati<strong>on</strong> in cereals, a greying ofchocolate and cocoa, and darkening of salt to yellow or red/brown.Sensory changes are highly variable and not always predictable. Just becausean ir<strong>on</strong> fortificant does not cause adverse sensory changes to a <strong>food</strong> product in<strong>on</strong>e situati<strong>on</strong>, does not necessarily mean that the same fortificant will not causea problem <strong>with</strong> the same <strong>food</strong> product in another situati<strong>on</strong>.Thus, having selecteda potential ir<strong>on</strong> fortificant, it is essential that its effects <strong>on</strong> the sensory propertiesof the <strong>food</strong> to which it is to be added are determined prior to use.5.1.5 Experience <strong>with</strong> ir<strong>on</strong> fortificati<strong>on</strong> of specific <strong>food</strong>sIr<strong>on</strong> fortificati<strong>on</strong> is already widely practised in many parts of the world. Forexample, more than 20 countries in Latin America have implemented mass ir<strong>on</strong>fortificati<strong>on</strong> programmes, most of which involve the fortificati<strong>on</strong> of wheat ormaize flours (237). Elsewhere, other frequently used <strong>food</strong> vehicles includecereal-based complementary <strong>food</strong>s, fish sauce, soy sauce and milk. Salt has alsobeen fortified <strong>with</strong> ir<strong>on</strong> in efficacy trials. Products derived from cereal flours(e.g. bread, cereal snacks and breakfast cereals) are also useful <strong>food</strong> vehicles, butthe amount of ir<strong>on</strong> provided via this route will depend <strong>on</strong> the quantity of <strong>food</strong>104
5. IRON, VITAMIN A AND IODINETABLE 5.2Suggested ir<strong>on</strong> fortificants for specific <strong>food</strong> vehiclesFood vehicleLow extracti<strong>on</strong> (white) wheatflour or degermed cornflourHigh extracti<strong>on</strong> wheat flour,corn flour, corn masa flourPastaRice aDry milkFluid milkCocoa productsSalt aSugar aSoy sauce, fish sauceJuice, soft drinksBouill<strong>on</strong> cubes aCereal-basedcomplementary <strong>food</strong>s bBreakfast cerealsFortificantDry ferrous sulfateFerrous fumarateElectrolytic ir<strong>on</strong> (×2 amount)Encapsulated ferrous sulfateEncapsulated ferrous fumarateSodium ir<strong>on</strong> EDTAFerrous fumarate (×2 amount)Encapsulated ferrous sulfate (×2 amount)Encapsulated ferrous fumarate (×2 amount)Dry ferrous sulfateFerric pyrophosphate (×2 amount)Ferrous sulfate plus ascorbic acidFerric amm<strong>on</strong>ium citrateFerrous bisglycinateMicr<strong>on</strong>ized ferric pyrophosphateFerrous fumarate plus ascorbic acidFerric pyrophosphate (×2 amount) plus ascorbic acidEncapsulated ferrous sulfateFerric pyrophosphate (×2 amount)Sodium ir<strong>on</strong> EDTASodium ir<strong>on</strong> EDTAFerrous sulfate plus citric acidFerrous bisglycinate, ferrous lactateMicr<strong>on</strong>ized ferric pyrophosphateMicr<strong>on</strong>ized ferric pyrophosphateFerrous sulfateEncapsulated ferrous sulfateFerrous fumarateElectrolytic ir<strong>on</strong> (×2 amount)All <strong>with</strong> ascorbic acid (≥2:1 molar ratio of ascorbic acid: Fe)Electrolytic ir<strong>on</strong> (×2 amount)EDTA, ethylenediaminetetraacetic acid.aTechnical problems, specifically sensory changes and/or segregati<strong>on</strong>, still exist <strong>with</strong> the ir<strong>on</strong>fortificati<strong>on</strong> of these <strong>food</strong> vehicles.bRecent evidence has indicated that infants may <strong>on</strong>ly absorb ferrous fumarate 25% as well asadults, so c<strong>on</strong>centrati<strong>on</strong>s of poorly soluble ir<strong>on</strong> compounds in complementary <strong>food</strong>s mayneed to be adjusted to allow for this.eaten and <strong>on</strong> the level of fortificati<strong>on</strong>. Ir<strong>on</strong> compounds suitable for the fortificati<strong>on</strong>of specific <strong>food</strong> vehicles are listed in Table 5.2.5.1.5.1 Wheat flourThe nutriti<strong>on</strong>al usefulness of ir<strong>on</strong> fortificati<strong>on</strong> of wheat flour has recently beenc<strong>on</strong>firmed in an efficacy study in Thailand (242). In that study the relative105
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSefficacy of electrolytic ir<strong>on</strong> as compared to ferrous sulfate was about 70% inwomen c<strong>on</strong>suming fortified wheat flour cookies, compared to 50% for H-reduced ir<strong>on</strong>. Based <strong>on</strong> this evidence, adding double the amount of electrolyticir<strong>on</strong> or H-reduced ir<strong>on</strong> as compared to ferrous sulfate, should give an equivalentefficacy to ferrous sulfate.Ferrous sulfate and elemental ir<strong>on</strong> powders have traditi<strong>on</strong>ally been used tofortify wheat and other cereal flours. Electrolytic ir<strong>on</strong> remains the preferredelemental ir<strong>on</strong> fortificant, however H-reduced ir<strong>on</strong> could also be c<strong>on</strong>sidered. Inadditi<strong>on</strong>, recent evidence from rat studies suggests that carb<strong>on</strong>yl ir<strong>on</strong> may be asgood as electrolytic ir<strong>on</strong> as a fortificant, however human efficacy studies are stillnecessary to c<strong>on</strong>firm this.Although ferrous sulfate has been successfully used for many years in Chile(where fortified flour is c<strong>on</strong>sumed <strong>with</strong>in 6–8 weeks of purchase), and ferrousfumarate has been employed in Venezuela and throughout Central America, inother countries the additi<strong>on</strong> of these ir<strong>on</strong> compounds to wheat flours has causedrancidity. This problem could be overcome by using encapsulated forms toimprove stability. Ferrous sulfate, and to a lesser extent ferrous fumarate, arealso suitable fortificants for pasta, which, because of its low moisture c<strong>on</strong>tent, isless susceptible than wheat flour to the development of rancidity.Although potentially useful for some high-phytate flours, NaFeEDTA has notbeen used widely in any large-scale ir<strong>on</strong> fortificati<strong>on</strong> programmes because ofreports that it interferes <strong>with</strong> the bread fermentati<strong>on</strong> process (243). However,China is currently introducing NaFeEDTA to fortify wheat in several provinces,and so far there have been no recorded problems. Although ascorbic acid is oftenadded to ir<strong>on</strong>-fortified <strong>food</strong>s in order to enhance absorpti<strong>on</strong> (see secti<strong>on</strong> 5.1.2.1),its usefulness in this respect in bread flours is limited by the fact that it isdestroyed by the acti<strong>on</strong> of heat during baking. Ascorbic acid is nevertheless frequentlyadded to flours, not so much to enhance ir<strong>on</strong> absorpti<strong>on</strong>, but rather asa raising agent.In its guidelines <strong>on</strong> ir<strong>on</strong> fortificati<strong>on</strong> of cereal-based staples, the SUSTAINTask Force (42) recommended the use of ferrous sulfate in preference, followedby ferrous fumarate, and lastly electrolytic ir<strong>on</strong> (but at twice the ir<strong>on</strong> c<strong>on</strong>centrati<strong>on</strong>of the other ir<strong>on</strong> compounds). In order to ensure the successful fortificati<strong>on</strong>of wheat flour and wheat flour products, it may be necessary for individualcountries to adopt different strategies to take account of differences in climate,wheat flour quality, processing methods and storage c<strong>on</strong>diti<strong>on</strong>s, as well as differencesin the main uses of flour (i.e. to make bread or other <strong>food</strong>s).5.1.5.2 MaizeIn general, maize flours are equally, if not more difficult, to fortify <strong>with</strong> ir<strong>on</strong> thanwheat flours. Lime-treated (nixtamalized) corn masa, a staple used to make106
5. IRON, VITAMIN A AND IODINEtortillas in much of Latin America, goes rancid when soluble ir<strong>on</strong> compounds,such as ferrous sulfate, are added to it. Further colour and texture changes occurduring the preparati<strong>on</strong> of tortillas. The difficulties are further compounded bythe fact that ir<strong>on</strong> absorpti<strong>on</strong> from corn masa is str<strong>on</strong>gly inhibited by its highphytate and high calcium c<strong>on</strong>tent. For these reas<strong>on</strong>s, ir<strong>on</strong> fortificati<strong>on</strong> of maizeflours has not been widely adopted, except in a number of Latin American countrieswhere the c<strong>on</strong>sumpti<strong>on</strong> of maize is high. In Venezuela, for example, ferrousfumarate mixed <strong>with</strong> elemental ir<strong>on</strong> is used to fortify maize flours.In view of its highly inhibitory nature (especially if it is not degermed), thePan American Health Organizati<strong>on</strong> (PAHO) recently recommended the use ofeither NaFeEDTA or ferrous fumarate (at twice the amount) for maize flourfortificati<strong>on</strong> (237). These recommendati<strong>on</strong>s have yet to be put into practice.Whether or not they are appropriate for maize meal that is used to prepare porridgealso needs to be evaluated. For maize flours that are not high in phyticacid (e.g. degermed) and are not lime-treated, the same ir<strong>on</strong> compounds as thoserecommended for the fortificati<strong>on</strong> of white wheat flour can be c<strong>on</strong>sidered (237).5.1.5.3 Cereal-based complementary <strong>food</strong>sComplementary <strong>food</strong>s (i.e. <strong>food</strong>s intended for infants during the weaningperiod) are usually based <strong>on</strong> dry cereals and c<strong>on</strong>sumed as a porridge or gruel<strong>with</strong> milk or water. Alternatively, they are based <strong>on</strong> blends of cereals andlegumes, which again can be made into a porridge or gruel <strong>with</strong> water.The additi<strong>on</strong>of ferrous sulfate, ferrous bisglycinate and other soluble ir<strong>on</strong> compounds tothese products can cause rancidity, and sometimes colour changes as well, particularlyif the porridges are fed <strong>with</strong> fruits. To overcome such problems, <strong>on</strong>eopti<strong>on</strong> would be to use encapsulated forms, such as ferrous sulfate. Althoughencapsulati<strong>on</strong> helps to prevent fat oxidati<strong>on</strong> during storage, the capsule isremoved by hot milk or water, and off-colours may still develop in the presenceof some fruits and vegetables.Another opti<strong>on</strong> is to use a less soluble ir<strong>on</strong> fortificant, such as ferrousfumarate or electrolytic ir<strong>on</strong> (but at a higher c<strong>on</strong>centrati<strong>on</strong>), both of which arecomm<strong>on</strong>ly used to fortify complementary <strong>food</strong>s. Ferric pyrophosphate isanother possibility, although it is rarely used in practice. If ferric pyrophosphatewere to be used to fortify complementary <strong>food</strong>s, it too should be added at twicethe c<strong>on</strong>centrati<strong>on</strong> (relative to ferrous sulfate). Recent evidence has indicated thatferrous fumarate may be less well absorbed in children than in adults (absorpti<strong>on</strong>of ir<strong>on</strong> from ferrous fumarate by children may <strong>on</strong>ly be 25% of that by adults)and so its use as a fortificant, or at least its level of additi<strong>on</strong>, may need to bere-evaluated (244).In order to enhance ir<strong>on</strong> absorpti<strong>on</strong>, ascorbic acid is usually added together<strong>with</strong> the ir<strong>on</strong> compound to complementary <strong>food</strong>s whenever possible (see secti<strong>on</strong>107
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS5.1.2.1). Ideally, ascorbic acid and ir<strong>on</strong> should be added in at least a 2 : 1 molarratio (ascorbic acid:ir<strong>on</strong>). Dry complementary <strong>food</strong>s should also be packagedin such as way as to minimize ascorbic acid degradati<strong>on</strong> during storage. Asdescribed above (see secti<strong>on</strong> 5.1.2.3), another useful way of optimizing ir<strong>on</strong>absorpti<strong>on</strong> from cereal-based <strong>food</strong>s is to degrade any phytic acid present <strong>with</strong>naturally-occurring cereal phytases (i.e. activate those already in the <strong>food</strong> bysoaking, germinating or fermenting) or by adding microbial phytases duringmanufacture. However, the additi<strong>on</strong> of phytases to processed <strong>food</strong>s has yet tobe attempted <strong>on</strong> a commercial scale.5.1.5.4 Dairy productsDried whole milk powders and dried or ready-to-feed milk-based infant formulascan be successfully fortified <strong>with</strong> ferrous sulfate (together <strong>with</strong> ascorbicacid to enhance absorpti<strong>on</strong>). In Chile, for example, ascorbic acid (700 mg/kg)and ir<strong>on</strong> (100 mg as ferrous sulfate/kg) are routinely added to dried milkpowders c<strong>on</strong>sumed by infants. In the case of soy formulas, it has been foundnecessary to use ferrous sulfate encapsulated <strong>with</strong> maltodextrin in order toprevent unwanted colour changes (i.e. darkening).Ferrous sulfate, and many other soluble ir<strong>on</strong> compounds, cannot be used tofortify liquid whole milk and other dairy products because they cause rancidityand off-flavours. Ferric amm<strong>on</strong>ium citrate (245), ferrous bisglycinate andmicr<strong>on</strong>ized ferric pyrophosphate are generally more suitable for this purpose.Ir<strong>on</strong> fortificants are best added after the milk has been homogenized and the fatinternalized in micelles, so as to help protect against oxidati<strong>on</strong>. Ferrous bisglycinateis widely used to fortify whole milk and dairy products in Brazil and Italy;micr<strong>on</strong>ized ferric pyrophosphate is added to dairy products in Japan (see alsosecti<strong>on</strong> 5.1.3.4).5.1.5.5 RiceThe fortificati<strong>on</strong> of rice grains presents a number of technical challenges. It canbe achieved, as is d<strong>on</strong>e in the United States, by coating the grain <strong>with</strong> an appropriateformulati<strong>on</strong>. Alternatively, a rice-based extruded grain that c<strong>on</strong>tains ahigh c<strong>on</strong>centrati<strong>on</strong> of ir<strong>on</strong> can be mixed <strong>with</strong> normal rice grains (usually at aratio of 1 : 200). Ferric pyrophosphate, added at a two-fold higher level, andmicr<strong>on</strong>ized, ferric pyrophosphate (0.5 micr<strong>on</strong>) have recently been recommendedfor adding to extruded artificial rice grains (246).Technical difficulties, combined <strong>with</strong> cultural preferences for specific types ofrice, mean that mass fortificati<strong>on</strong> of rice, although desirable, remains problematic.The fact that in most of the big rice-producing countries, producti<strong>on</strong> takesplace in thousands of small mills, also creates problems for mass rice fortificati<strong>on</strong>.Not <strong>on</strong>ly are smaller mills sensitive to small increases in costs, the sheer108
5. IRON, VITAMIN A AND IODINEnumber of them makes it difficult to maintain adequate quality c<strong>on</strong>trol programmes.Although the extruded grains have found some applicati<strong>on</strong> in targeted<strong>food</strong> fortificati<strong>on</strong> programmes, such as school feeding programmes, much moreresearch and development is required before mass rice fortificati<strong>on</strong> programmescan be implemented <strong>on</strong> a wider scale.5.1.5.6 Cocoa productsAs cocoa is naturally high in phenolic compounds, the additi<strong>on</strong> of ferrous sulfateand other water-soluble ir<strong>on</strong> compounds tends to cause colour changes incocoa-based products (247). Ferrous fumarate is a useful alternative for someproducts, but grey or blue/grey colours are still a problem for chocolate drinks,especially if boiling water is used to make up the drink (227). Furthermore, thecurrently available encapsulated ir<strong>on</strong> compounds are not useful for chocolatedrink fortificati<strong>on</strong> as the capsules are removed by heat either during productmanufacture or during preparati<strong>on</strong> of the drink.Ferric pyrophosphate, ferric saccharate or ferric orthophosphate are usuallyused to fortify cocoa products as these tend to produce fewer off-colours.However, relative to ferrous sulfate, larger amounts of these ir<strong>on</strong> compoundswould need to be added to allow for their lower absorpti<strong>on</strong>. Ascorbic acid additi<strong>on</strong>is also required (in at least a 2 : 1 molar ratio) in order to offset the inhibitoryeffects of cocoa phenolics <strong>on</strong> ir<strong>on</strong> absorpti<strong>on</strong> (227,248).5.1.5.7 Soy sauce and fish sauceSodium ir<strong>on</strong> EDTA has proved to be a useful fortificant for both fish sauce andsoy sauce (see also secti<strong>on</strong> 1.3.1). Studies have dem<strong>on</strong>strated that absorpti<strong>on</strong> ofir<strong>on</strong> by human subjects fed NaFeEDTA-fortified fish or soy sauce added to ricemeals is similar to that from the same meals to which ferrous sulfate-fortifiedsauces had been added (249). The ir<strong>on</strong> status of ir<strong>on</strong>-deficient Vietnamesewomen improved significantly following regular intakes of NaFeEDTA-fortifiedfish sauce over a period of 6 m<strong>on</strong>ths (28) (see also secti<strong>on</strong> 1.3.1.1). Similarly, intrials c<strong>on</strong>ducted in China, NaFeEDTA soy sauce, providing 20 mg ir<strong>on</strong> per day,significantly improved the ir<strong>on</strong> status of anaemic adolescents (250). Large-scaleeffectiveness studies of soy sauce fortificati<strong>on</strong> <strong>with</strong> NaFeEDTA are currentlyunderway in both Viet Nam and China.Until very recently, NaFeEDTA has been the preferred ir<strong>on</strong> fortificantfor soy and fish sauces because most of the potential alternatives (i.e. othersoluble ir<strong>on</strong> compounds) cause peptide precipitati<strong>on</strong> during storage. However,latterly ferrous sulfate stabilized <strong>with</strong> citric acid has been successfully used tofortify fish sauce in Thailand, and may offer a less expensive alternative toNaFeEDTA.109
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS5.1.5.8 SaltThe success of salt iodizati<strong>on</strong> programmes (see secti<strong>on</strong> 5.3.2.1) has led severalcountries to c<strong>on</strong>sider using salt as a vehicle for ir<strong>on</strong> fortificati<strong>on</strong>. In practice, thismeans the double fortificati<strong>on</strong> of salt, i.e. <strong>with</strong> ir<strong>on</strong> and iodine. Promisingapproaches that are already being tested include the additi<strong>on</strong> of encapsulatedferrous fumarate, encapsulated ferrous sulfate (see secti<strong>on</strong> 1.3.2.3) or ferricpyrophosphate (at twice the c<strong>on</strong>centrati<strong>on</strong>). Encapsulati<strong>on</strong> is necessary asferrous sulfate, ferrous fumarate and other soluble ir<strong>on</strong> compounds very quicklycause a yellow or red/brown discolorati<strong>on</strong> in the moist, low quality salt that iscurrently used in many developing countries. The main disadvantage of theencapsulati<strong>on</strong> opti<strong>on</strong>s is the increase in the price of the fortified product, whichcan be by as much as 30%.5.1.6 Safety issuesC<strong>on</strong>cern has been raised about increased ir<strong>on</strong> intakes, particularly in terms ofthe potential effects <strong>on</strong> infecti<strong>on</strong> rates and <strong>on</strong> the risk of cardiovascular diseaseand cancer. Much of this c<strong>on</strong>cern, however, relates to the use of pharmaceuticalir<strong>on</strong> supplements and not to fortified <strong>food</strong>s.A recent review of interventi<strong>on</strong> studies <strong>with</strong> ir<strong>on</strong>-fortified milk or cereals, c<strong>on</strong>cludedthat ir<strong>on</strong> fortificati<strong>on</strong> did not increase infectious morbidity in childrenunder 18 m<strong>on</strong>ths of age (251). Studies in Chile (252), Hungary (253) and SouthAfrica (254) reported that ir<strong>on</strong> added to milk formula had no influence <strong>on</strong>infectious outcome. Only <strong>on</strong>e study, c<strong>on</strong>ducted in a poor community in Chile,reported an increase in episodes of diarrhoea in young infants fed ir<strong>on</strong>-fortifiedformula (255). On balance, studies have indicated that ir<strong>on</strong> fortificati<strong>on</strong> of milkformula is safe (251).It has been suggested that higher levels of ir<strong>on</strong> intake and elevated body storesare potential risk factors for both cor<strong>on</strong>ary heart disease (CHD) and cancer.Results from studies carried out over the last 10 years to test this hypothesis are,however, inc<strong>on</strong>clusive. The associati<strong>on</strong> between serum ferritin and risk of CHDhas been examined in at least 12 studies, but a meta-analysis of such evidencefailed to establish a str<strong>on</strong>g relati<strong>on</strong>ship between the two (256). Inflammatoryresp<strong>on</strong>se is an important risk factor for CHD and also increases serum ferritin,which might explain why an associati<strong>on</strong> between the risk of CHD and increasedserum ferritin is sometimes observed.Possible links between cancer and ir<strong>on</strong> intake or ir<strong>on</strong> status have been thesubject of <strong>on</strong>ly a few studies, but are largely unsubstantiated. It has been hypothesizedthat the presence of unabsorbed fortificant ir<strong>on</strong> in the body, much ofwhich reaches the col<strong>on</strong>, leads to free radical generati<strong>on</strong> that damages the col<strong>on</strong>mucosa (257). However, ir<strong>on</strong> is highly insoluble at the pH of the col<strong>on</strong>, andalthough unabsorbed ferrous sulfate can increase free radical generati<strong>on</strong> in the110
5. IRON, VITAMIN A AND IODINEstool (257), there is no evidence to suggest that the free radicals survive l<strong>on</strong>genough to cause tissue damage. The finding that serum transferrin was higherin men who developed col<strong>on</strong> cancer (258) was not c<strong>on</strong>firmed when the followupwas extended to 17 years.Summary: ir<strong>on</strong> fortificati<strong>on</strong>■ For most <strong>food</strong> vehicles, the recommended ir<strong>on</strong> fortificants, in order of preference,are: ferrous sulfate, ferrous fumarate, encapsulated ferrous sulfate or fumarate,electrolytic ir<strong>on</strong> (at twice the amount), and ferric pyrophosphate (at twice theamount).■ The co-additi<strong>on</strong> of ascorbic acid in a 2 : 1 molar ratio is recommended in order toenhance ir<strong>on</strong> absorpti<strong>on</strong>. This applies to infant <strong>food</strong>s and market-driven <strong>food</strong>s. Inthe case of high phytic acid <strong>food</strong>s, the molar ratio (ascorbic acid:ir<strong>on</strong>) can beincreased to 4 : 1.■ NaFeEDTA is recommended for the mass fortificati<strong>on</strong> of high-phytate cereal floursand for sauces <strong>with</strong> a high peptide c<strong>on</strong>tent (e.g. fish sauce, soy sauce).■ For liquid milk products, ferrous bisglycinate, micr<strong>on</strong>ized ferric pyrophosphate andferric amm<strong>on</strong>ium citrate are the most appropriate fortificants.5.2 Vitamin A and β-carotene5.2.1 Choice of vitamin A fortificantThe choice of a vitamin A fortificant is largely governed by the characteristicsof the <strong>food</strong> vehicle, as well as various technological, regulatory and religious c<strong>on</strong>siderati<strong>on</strong>s.As preformed vitamin A (retinol) is an unstable compound, in commercialpreparati<strong>on</strong>s it is esterified, usually <strong>with</strong> palmitic or acetic acid, to themore stable corresp<strong>on</strong>ding esters. Retinyl acetate and retinyl palmitates, al<strong>on</strong>g<strong>with</strong> provitamin A (β-carotene), are thus the main commercial forms of vitaminA that are available for use as <strong>food</strong> fortificants. The intense orange colour of β-carotene makes it unsuitable for use as a fortificant in many <strong>food</strong>s, but it is widelyused to give an orange-yellow colour to margarines and beverages.Since vitamin A is fat-soluble, it is easily added to fat-based or oily <strong>food</strong>s.When the <strong>food</strong> vehicle is either dry or a water-based liquid, an encapsulatedform of the vitamin is needed. Based <strong>on</strong> this distincti<strong>on</strong>, vitamin A fortificantscan be divided into two categories:• Oily forms that can be incorporated directly into fat-based <strong>food</strong>s or emulsifiedinto water-based <strong>on</strong>es (e.g. milk).• Dry forms that can be dry mixed into <strong>food</strong>s or dispersed in water, depending<strong>on</strong> whether they are cold water dispersible or n<strong>on</strong>-cold water dispersible.111
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 5.3Commercially available forms of vitamin A, their characteristics and their mainapplicati<strong>on</strong>sProduct Characteristics Applicati<strong>on</strong>(s)Oily vitamin A Retinol ester of acetic acid which Fortificati<strong>on</strong> of fat-based <strong>food</strong>s,acetate may be stabilized <strong>with</strong> especially margarine and dairyantioxidantsproductsOily vitamin A Retinol ester of palmitic acid Fortificati<strong>on</strong> of fat-based <strong>food</strong>s,palmitate which may be stabilized <strong>with</strong> especially margarine and dairyantioxidantsproductsOily vitamin A Retinol ester and cholecalciferol Fortificati<strong>on</strong> of fat-based <strong>food</strong>s,palmitate or mix, stabilized <strong>with</strong> antioxidants especially margarine and dairyacetate <strong>with</strong>products where the combinati<strong>on</strong>vitamin D 3of both vitamins is requiredDry vitamin A Vitamin A embedded in a water- Fortificati<strong>on</strong> of dry <strong>food</strong> products,palmitate or soluble matrix (e.g. gelatin, gum (i.e. flour and dry milk, beverageacetate acacia, starch) and stabilized powders) and fortificati<strong>on</strong> of water<strong>with</strong>antioxidantsbased <strong>food</strong>sDry vitamin A Vitamin A and vitamin D 3 Fortificati<strong>on</strong> of dry <strong>food</strong> products,palmitate or embedded in a water-soluble (i.e. flour and dry milk, beverageacetate <strong>with</strong> matrix (e.g. gelatin, gum acacia, powders) and fortificati<strong>on</strong> of watervitaminD 3 starch) and stabilized <strong>with</strong> based <strong>food</strong>santioxidantsSource: Hector Cori, pers<strong>on</strong>al communicati<strong>on</strong>, 2004.Pure vitamin A and β-carotene in soluti<strong>on</strong> are unstable when exposed to ultravioletlight, oxygen or air. Thus all forms of vitamin A – oily or dried – are protected<strong>with</strong> antioxidants to prol<strong>on</strong>g their shelf-life. The use of airtight packagingprovides further protecti<strong>on</strong>. For example, the loss of vitamin A in sealed cansof oil is minimal, but losses from fortified cereals, fortified sugar or oil can beas high as 40%, depending <strong>on</strong> ambient c<strong>on</strong>diti<strong>on</strong>s and storage times (259–261).Opaque packaging is indispensable for maintaining stability in vitamin A-fortified oils.The characteristics and applicati<strong>on</strong>s of the various forms of vitamin A arelisted in Table 5.3. Each formulati<strong>on</strong> includes stabilizers, and each is compatible<strong>with</strong> existing <strong>food</strong> regulati<strong>on</strong>s (e.g. c<strong>on</strong>tain permitted antioxidants) and/orreligious requirements (e.g. Kosher, Halal). The fat-soluble forms of retinol areabout <strong>on</strong>e half to <strong>on</strong>e third as expensive as the dry forms. Appropriate vitaminA fortificants for specific <strong>food</strong>s are given in Table 5.4.5.2.2 Experience <strong>with</strong> vitamin A fortificati<strong>on</strong> of specific <strong>food</strong>sOf the <strong>food</strong> vehicles suitable for mass fortificati<strong>on</strong>, margarine is the <strong>on</strong>e thatis most frequently associated <strong>with</strong> vitamin A. In both industrialized and112
5. IRON, VITAMIN A AND IODINETABLE 5.4Vitamin A fortificants and their suitability as fortificants for specific <strong>food</strong>vehiclesFood vehicle Form of vitamin A StabilityCereal flours Retinyl acetate or retinyl palmitate (dry stabilized Fairforms)Fats and oils β-carotene and retinyl acetate or retinyl palmitate Good(oil-soluble)Sugar Retinyl palmitate (water dispersible forms) FairMilk powder Retinyl acetate or palmitate (dry water dispersible Goodforms)Liquid milk Retinyl acetate (preferred) or palmitate (oily form, Good/fair dependingemulsified)<strong>on</strong> packagingInfant formula Retinyl palmitate (water dispersible beadlets) GoodSpreads Retinyl acetate or palmitate (oily form) GoodSource: Hector Cori, pers<strong>on</strong>al communicati<strong>on</strong>, 2004.developing countries, vegetable oils are also used, and, in recent years, cerealflours have increasingly been fortified <strong>with</strong> vitamin A in several parts of theworld. In parts of Central America, sugar is often the preferred <strong>food</strong> vehicle forvitamin A. The amount and forms of vitamin A used in a selecti<strong>on</strong> of <strong>food</strong> fortificati<strong>on</strong>programmes are detailed in Table 5.5. It is estimated that about 90%of fortificant vitamin A will usually be absorbed (262).5.2.2.1 Oils and margarineThere are two reas<strong>on</strong>s why margarines and oils are the ideal <strong>food</strong>s for vitaminA fortificati<strong>on</strong>. Not <strong>on</strong>ly is the oil-soluble form of the vitamin the cheapest available,but the oil protects the vitamin A from oxidati<strong>on</strong> during storage and sofacilitates absorpti<strong>on</strong> of the vitamin (264). The vitamin A fortificati<strong>on</strong> of margarineshas a relatively l<strong>on</strong>g history, having been introduced in some countriesas early as the 1920s, following the realizati<strong>on</strong> that the replacement of butter<strong>with</strong> margarine in the diet was causing widespread xerophthalmia in children(265). Vitamin A fortificati<strong>on</strong> of margarine in Newfoundland, Canada, forexample, resulted in a marked improvement in vitamin A status (266). Likewise,in India, a hydrogenated oil (vanaspati), which is used as an alternative to ghee,has been fortified <strong>with</strong> vitamin A since 1953 (267).Although the technology for adding vitamin A to oils is simple and inexpensive,and oils are widely used, the fortificati<strong>on</strong> of oils <strong>with</strong> this vitamin is relativelyrare, at least compared <strong>with</strong> that of margarines. The fortificati<strong>on</strong> of oils isthus a potentially useful means of expanding the present range of vitamin A-fortified <strong>food</strong>s. Stability may be a problem in some settings; experimental studieshave shown that when vitamin A is added to soybean oil in sealed cans, the113
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 5.5Examples of vitamin A fortificati<strong>on</strong> programmesFood item Country or Amount of Form of vitamin A Amount of <strong>food</strong> c<strong>on</strong>sumed C<strong>on</strong>tributi<strong>on</strong> toprogramme retinol added added (g/day) recommended(mg/kg) daily intake (%)Margarine Philippines 25 Retinyl palmitate (oil) 24 (preschool-aged children) 150 aMargarine Various 1–15 Retinyl palmitate (oil) 15 2–40 aVegetable oil (PL-480) US Food Aid 18 Retinyl palmitate (oil) 16 50 aHydrogenated fat India, Pakistan 7.5 Retinyl palmitate (oil) 0.3–17 0.4–21 aMaize flour Venezuela 2.7 Retinyl palmitate (dry) 80 30Wheat flour Philippines 4.5 Retinyl palmitate (dry) 40 (bread) 19 aWheat flour US Food Aid 6.6–7.9 Retinyl palmitate (dry) 75 80–100 aSugar Guatemala 15 Retinyl palmitate (dry) 30–120 (average, 60) (adults) 45–180 (adults)20–30 (young children) 30 (
5. IRON, VITAMIN A AND IODINEvitamin was stable for up to 9 m<strong>on</strong>ths. However, although less than 15% of thevitamin A was lost during boiling or pressure cooking of rice or beans, about60% was lost when the oil was reused several times for frying (260).There has been little systematic evaluati<strong>on</strong> of the effectiveness of margarineand oil fortificati<strong>on</strong>, although historical data from Europe suggest that it hasbeen effective in c<strong>on</strong>trolling vitamin A deficiency. In the Philippines, c<strong>on</strong>sumpti<strong>on</strong>of “Star margarine”, which is fortified <strong>with</strong> 25 mg vitamin A/kg plus 3.5 mgβ-carotene/kg, significantly reduced the prevalence of low serum retinol. PL-480vegetable oil, which is distributed in emergency feeding programmes, is intendedto provide about 50% of the recommended daily intake of vitamin A for an adultmale (assuming a daily intake of 16 g per pers<strong>on</strong>) (see Table 5.5). The stabilityof vitamin A in previously unopened pails of PL-480 oil is excellent, althoughup to 30% losses can occur in opened pails after 30 days of storage. Vitamin Aretenti<strong>on</strong> in the oil is also good, <strong>with</strong> <strong>on</strong>ly a 10% loss after 30 minutes of heating(268).5.2.2.2 Cereals products and floursWholegrain cereals and flours c<strong>on</strong>tain negligible, if any, amounts of intrinsicvitamin A. Flours are, nevertheless, potentially good vehicles for vitamin Afortificati<strong>on</strong>, because dry forms of vitamin A can easily be mixed in <strong>with</strong> otheradditives. Despite this, cereal flours are not fortified <strong>with</strong> vitamin A in mostindustrialized countries, because, for historical reas<strong>on</strong>s, margarines are thepreferred vehicle and, furthermore, because vitamin A deficiency is no l<strong>on</strong>ger asignificant problem. The United States Title II Food Aid Program has beenfortifying wheat-soy and corn-soy blends <strong>with</strong> vitamin A for about 30 years;working <strong>on</strong> the assumpti<strong>on</strong> that the recipients are likely to be highly dependent<strong>on</strong> these fortified <strong>food</strong>s for their vitamin A needs, it adds sufficient amounts toprovide 100% of the recommended daily intake of this particular vitamin (269).However, between 30% and 50% of the vitamin A that is added to the blendedcereals is lost in shipping and storage (268,270).Wheat flour is fortified <strong>with</strong> 4.5 mg retinol/kg in some mills in the Philippines,a practice which provides an average c<strong>on</strong>centrati<strong>on</strong> in bread of 2.2µg retinol/g(Table 5.5). This supplies about 33% of the recommended daily intake forvitamin A for school-age children. At this level of fortificati<strong>on</strong>, retinol liver storesin deficient children were significantly increased at the end of a 30-week efficacytrial (33) (see also secti<strong>on</strong> 1.3.1.2).Pre-cooked maize flour has been fortified <strong>with</strong> vitamin A in Venezuela since1993 (Table 5.5). A fortificati<strong>on</strong> level of 2.7 mg/kg and an intake of 80 gflour/day supplies about 40% of an average family’s recommended intake (271).However, the impact of maize fortificati<strong>on</strong> <strong>on</strong> the vitamin A status of the generalpopulati<strong>on</strong> is not known.115
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS5.2.2.3 SugarIn the 1970s, vitamin A fortificati<strong>on</strong> of sugar was implemented in Costa Ricaand Guatemala, because it was the <strong>on</strong>ly centrally processed <strong>food</strong> vehicle thatwas c<strong>on</strong>sumed in adequate amounts by the poorer segments of the populati<strong>on</strong>.Such programmes ceased for a time during the 1980s but are again functi<strong>on</strong>ingGuatemala, and also in El Salvador, H<strong>on</strong>duras and Nicaragua where theyreceive str<strong>on</strong>g support from the sugar industry (272). An early evaluati<strong>on</strong> ofvitamin A fortificati<strong>on</strong> of sugar in Guatemala showed that it is an effective strategyfor improving vitamin A status and for increasing the amount of the vitaminin breast milk of lactating mothers (273) (see also secti<strong>on</strong> 1.3.2.4). Fortified sugarin Guatemala provides children <strong>with</strong> about <strong>on</strong>e third of their recommendedintake of vitamin A (274) (Table 5.5). Sugar fortificati<strong>on</strong> is now being implementedin other parts of the world, such as Zambia.Large quantities of sugar are used in a wide range of commercial <strong>food</strong>s, suchas c<strong>on</strong>fecti<strong>on</strong>ery and soft drinks. Retinol in fortified unrefined sugar survivesthe baking process but is lost during soft-drink producti<strong>on</strong> (in fortified unrefinedsugar <strong>on</strong>ly <strong>on</strong>e third of the initial level remains after 2 weeks of storage).Depending <strong>on</strong> the level of soft drink producti<strong>on</strong>, these losses can have importantcost implicati<strong>on</strong>s and it may be appropriate for the soft drink sector to beexempt from having to use fortified sugar (275).5.2.2.4 RiceGiven that rice is an important staple in many countries where the prevalenceof vitamin A deficiency is high, vitamin A fortificati<strong>on</strong> of rice has the potentialto be an effective public health strategy for the eliminati<strong>on</strong> of VAD. However, asis the case <strong>with</strong> ir<strong>on</strong>, for technical reas<strong>on</strong>s, rice fortificati<strong>on</strong> <strong>with</strong> vitamin A isstill at an experimental stage. Again, the predominance of small-scale mills inthe rice-producing countries hinders the implementati<strong>on</strong> of fortificati<strong>on</strong> programmesusing rice as the chosen <strong>food</strong> vehicle.5.2.2.5 Other <strong>food</strong>s and beveragesOther <strong>food</strong>s that have been fortified successfully <strong>with</strong> preformed or provitaminA include:—dry milk;— complementary <strong>food</strong>s for infants and young children;— biscuits and beverages, which are sold commercially or used in schoolfeeding programmes such as those implemented in Ind<strong>on</strong>esia, Mexico andother countries in Central America (276), (277), Peru (278) and SouthAfrica (34);116
5. IRON, VITAMIN A AND IODINE— instant noodles (in Thailand), the vitamin A (and elemental ir<strong>on</strong> 1 ) beingsupplied in the spices that are provided in a separate sachet (279);—yoghurt (worldwide) (280).5.2.3 Safety issuesAdverse physiological effects have been associated <strong>with</strong> both acute hypervitaminosisA and chr<strong>on</strong>ic high intake. The routine c<strong>on</strong>sumpti<strong>on</strong> of large amountsof vitamin A over a period of time can result in a variety of toxic symptomsincluding liver damage, b<strong>on</strong>e abnomalities and joint pain, alopecia, headaches,vomiting and skin desquamati<strong>on</strong> (93).For l<strong>on</strong>g-term daily intakes, the United States Institute of Medicine’s Foodand Nutriti<strong>on</strong> Board (IOM/FNB) have defined Tolerable Upper Intake Levels(ULs) for vitamin A, as follows (91):— 600µg/day for children
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSSummary: vitamin A fortificati<strong>on</strong>■ A variety of oily and dry forms of the retinol esters, retinyl acetate and retinyl palmitate,are available for <strong>food</strong> fortificati<strong>on</strong> purposes. The dry forms are usually gelatin-, starch- or gum-coated and all forms c<strong>on</strong>tain antioxidants.■ Absorpti<strong>on</strong> of all forms is good (around 90%) but losses of vitamin A during processing,storage and <strong>food</strong> preparati<strong>on</strong> may be high.■ Vitamin A fortificati<strong>on</strong> of margarine and sugar has been shown to be efficacious.Vegetable oils and cereal flours are also c<strong>on</strong>sidered to be useful fortificati<strong>on</strong> vehicles.■ Adverse health effects have been associated <strong>with</strong> acute and chr<strong>on</strong>ic high intakesof retinol (mainly through supplementati<strong>on</strong>) but not <strong>with</strong> high intakes of the provitaminA carotenoids.TABLE 5.6Iodine fortificants: chemical compositi<strong>on</strong> and iodinec<strong>on</strong>tentFortificant Formula Iodine c<strong>on</strong>tent (%)Calcium iodide CaI 2 86.5Calcium iodate Ca(IO 3 ) 2 .6H 2 O 65.0Potassium iodide KI 76.5Potassium iodate KIO 3 59.5Sodium iodide NaI.2H 2 O 68.0Sodium iodate NaIO 3 64.05.3 Iodine5.3.1 Choice of iodine fortificantThere are two chemical forms of iodine that are suitable for use as <strong>food</strong> fortificants,namely, iodate and iodide. They are usually added as the potassium salt,but sometimes as the calcium or sodium salt (Table 5.6).Potassium iodide has been used as an additive in bread and salt for about 80years, and potassium iodate for about 50 years. Iodates are less soluble in waterthan the iodides, more resistant to oxidati<strong>on</strong> and evaporati<strong>on</strong>, and being morestable under adverse climatic c<strong>on</strong>diti<strong>on</strong>s, do not require the co-additi<strong>on</strong> of stabilizers.Although more expensive, potassium iodate is thus preferred to potassiumiodide, especially in hot and humid climates, and is recommended asan additive for many <strong>food</strong>s, including salt (282,283). For historical reas<strong>on</strong>s,however, countries in Europe and North America still use potassium iodide,while most countries <strong>with</strong> tropical climates use potassium iodate. Losses of118
5. IRON, VITAMIN A AND IODINEiodine because of iodide oxidati<strong>on</strong> are increased by moisture, humidity, exposureto heat and sunlight, or by impurities in the salt to which it is added.5.3.2 Experience <strong>with</strong> iodine fortificati<strong>on</strong> of specific <strong>food</strong>s5.3.2.1 SaltSalt is the most widely used <strong>food</strong> vehicle for iodine fortificants. Indeed, universalsalt iodizati<strong>on</strong> (USI), that is, the iodizati<strong>on</strong> of all salt for human (<strong>food</strong> industryand household) and livestock c<strong>on</strong>sumpti<strong>on</strong>, is the strategy recommended byWHO for the c<strong>on</strong>trol of iodine deficiency disorders (284). The choice of thisstrategy is based <strong>on</strong> the following factors:— salt is <strong>on</strong>e of the few commodities c<strong>on</strong>sumed by every<strong>on</strong>e;— salt c<strong>on</strong>sumpti<strong>on</strong> is fairly stable throughout the year;— salt producti<strong>on</strong> is usually limited to a few geographical areas;— salt iodizati<strong>on</strong> technology is easy to implement and available at reas<strong>on</strong>ablecost throughout the developing world (0.2–0.3 US cents/kg, or 1 US centper pers<strong>on</strong>/year);— the additi<strong>on</strong> of iodine to salt does not affect its colour, taste or odour;— the quality of iodized salt can be m<strong>on</strong>itored at the producti<strong>on</strong>, retail andhousehold levels.The mining of solid rock deposits is the main source of salt in Australia, Europeand North America. Elsewhere, i.e. in Africa, Asia and South America, solarevaporati<strong>on</strong> of either sea water, lake or underground brines is the main source.After extracti<strong>on</strong>, crude salt is refined so that its purity increases from 85–95%NaCl to 99% NaCl. Specificati<strong>on</strong>s for the physical characteristics and chemicalcompositi<strong>on</strong> required for <strong>food</strong> grade salt are laid down in the CodexAlimentarius (285).Iodine is usually added to salt after the salt has been refined and dried, by<strong>on</strong>e of two main techniques. In the wet method, a soluti<strong>on</strong> of potassium iodate(KIO 3 ) is either dripped or sprayed at a uniform rate <strong>on</strong>to salt passing by <strong>on</strong> ac<strong>on</strong>veyor belt. The technique is particularly cost-effective. For instance, inSwitzerland, a single c<strong>on</strong>veyor belt and sprayer produces enough salt for 6milli<strong>on</strong> people at a cost of 1 US$ per 100 kg salt or 7 US cents per pers<strong>on</strong> peryear (286). The alternative method, the dry method, involves sprinkling potassiumiodide powder (KI) or potassium iodate (KIO 3 ) over the dry salt. Thistechnique is more demanding, in that it requires a salt made of small homogenouscrystals and the thorough mixing of the salt after additi<strong>on</strong> of the iodinecompound to ensure an even distributi<strong>on</strong> of iodine. Poor mixing is a major cause119
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSof inappropriate salt iodizati<strong>on</strong>. Technical informati<strong>on</strong> <strong>on</strong> the salt iodizati<strong>on</strong>process is available elsewhere (287).The stability of iodine in salt depends <strong>on</strong> the water c<strong>on</strong>tent, acidity and purityof the salt to which it is added. In order to reduce iodine losses during storage,the iodized salt must be as pure and as dry as possible, and it must be appropriatelypackaged. Iodine tends to migrate from the top to the bottom of a c<strong>on</strong>tainerwhen the water c<strong>on</strong>tent is too high. It will evaporate if the acidity is toohigh. Losses also tend to occur when packaging <strong>with</strong> impervious linings is used;as the packaging becomes damp, the iodide migrates from the salt to the fabric,and then evaporates. This is less likely to happen <strong>with</strong> potassium iodate becausethe iodates are less soluble and more resistant to oxidati<strong>on</strong>. Types of packagingthat help to prevent iodine losses include high density polyethylene bags that areeither laminated <strong>with</strong> low density polyethylene or lined <strong>with</strong> a c<strong>on</strong>tinuous filmthat is resistant to puncture. In a multi-country study of iodine losses from salt,high humidity combined <strong>with</strong> porous packing (such as jute bags), caused a30–80% loss of iodine over a period of 6 m<strong>on</strong>ths (288).Because salt iodizati<strong>on</strong> is cheap and easy to implement, great strides in saltiodizati<strong>on</strong> programmes have been made in a relatively short period of time(Table 5.7). During the 10-year period, 1989 to 1999, the proporti<strong>on</strong> of householdsc<strong>on</strong>suming iodized salt increased from 10% to 68% and by 1999, of 130countries affected by iodine deficiency, 98 had in place legislati<strong>on</strong> requiring theiodizati<strong>on</strong> of salt (284). Several factors have limited progress towards the goalof USI; these include difficulties in enforcing legislati<strong>on</strong> <strong>on</strong> iodized salt; problemscaused by having a high number of small-scale salt producers and theabsence of an operati<strong>on</strong>al m<strong>on</strong>itoring system. The existence of pockets of populati<strong>on</strong>sliving in remote areas that cannot easily access iodized salt is anotherfactor which can hinder the effective implementati<strong>on</strong> of salt iodizati<strong>on</strong> programmesand their sustainability in some countries. In order to assist countriesTABLE 5.7Progress towards universal salt iodizati<strong>on</strong> in WHO regi<strong>on</strong>s, status as of 1999WHO regi<strong>on</strong> Coverage (% of households) No. of countries <strong>with</strong> legislati<strong>on</strong><strong>on</strong> iodized saltAfrica 63 34Americas 90 17South-East Asia 70 7Europe 27 20Eastern Mediterranean 66 14Western Pacific 76 6Total 68 98Sources: adapted from references (284,289).120
5. IRON, VITAMIN A AND IODINEdevelop and sustain effective salt iodizati<strong>on</strong> programmes, several internati<strong>on</strong>alorganizati<strong>on</strong>s, including WHO, have jointly established a mechanism forstrengthening nati<strong>on</strong>al capacity in activities that support salt iodizati<strong>on</strong>, in particular,quality assurance and m<strong>on</strong>itoring. The work of the Internati<strong>on</strong>alResource Laboratory for Iodine network (IRLI), which includes trainingand technology transfer and informati<strong>on</strong> sharing, is outlined in more detail inAnnex B.5.3.2.2 BreadFrom a technical point of view, bread is a good vehicle for iodine and has beenshown to be an effective way of ensuring a c<strong>on</strong>stant supply of dietary iodine. Ithas been used in a few European countries where bread is a staple <strong>food</strong>, suchas Russia (290,291), and in Tasmania.The main carrier for iodine in the Netherlandsis the salt added to bread, i.e. baker’s salt, which has been enriched <strong>with</strong>iodine since 1942. In recent years, the potassium iodide c<strong>on</strong>tent of Dutch baker’ssalt has been increased.5.3.2.3 WaterBecause water is c<strong>on</strong>sumed daily, it too has the potential to be a useful vehiclefor iodine fortificati<strong>on</strong>. Its major limitati<strong>on</strong>, compared <strong>with</strong> salt, is that sourcesof drinking water are so numerous and ubiquitous that iodizati<strong>on</strong> would be difficultto c<strong>on</strong>trol. Moreover, iodine has limited stability in water (no l<strong>on</strong>ger than24 hours) such that c<strong>on</strong>tinuous daily dosing of the water supply would be necessary.Although the use of water as a vehicle for iodine fortificati<strong>on</strong> is technicallymore difficult than the use of salt, there are certain c<strong>on</strong>diti<strong>on</strong>s where wateriodizati<strong>on</strong> could be a suitable method for the correcti<strong>on</strong> of iodine deficiency.The simplest way of fortifying water <strong>with</strong> iodine is to add a c<strong>on</strong>centratediodine soluti<strong>on</strong> (as potassium iodide or iodate) in a dropwise fashi<strong>on</strong> until aspecified c<strong>on</strong>centrati<strong>on</strong> in the water c<strong>on</strong>tained in a given vessel is reached. Thismethod is widely used in schools in northern Thailand (292). Alternatively, inthe case of hand pumps and open wells, iodine in porous polymer c<strong>on</strong>tainerscan be introduced into the water supply. The porous c<strong>on</strong>tainers allow the slowrelease of potassium iodide soluti<strong>on</strong> into the water supply. However, such c<strong>on</strong>tainershave a limited shelf-life and must be changed every year. Such practiceshave been successful in several parts of the world; in Africa, in the CentralAfrican Republic, Mali (293) and Sudan (294), in Asia, in the central Asianrepublics, Malaysia (295) and Thailand and in Europe, in Italy (Sicily). In mostsettings, the limiting factor, especially in terms of cost-effectiveness, is that thewhole populati<strong>on</strong> and the livestock need to use the iodized water supply pointto benefit from iodizati<strong>on</strong> (296). A third opti<strong>on</strong>, which is suitable for piped watersupplies, is to divert some of the piped water through a canister packed <strong>with</strong>121
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSiodine crystals, and then reintroduce this iodized water back into the main watersupply. The direct additi<strong>on</strong> of an iodine soluti<strong>on</strong> to freshwater supplies has alsobeen attempted. For instance, a 5% potassium iodate soluti<strong>on</strong> was introducedinto the single river which supplied water to an isolated populati<strong>on</strong> in China fora period of 12–24 days (297). The result was an improvement in urinary iodineof children, and a relatively stable increase in soil iodine.A review of the efficacy and cost-effectiveness of the different proceduresused to iodize water c<strong>on</strong>cluded that while efficacious for the most part, there isno doubt that the cost, and the m<strong>on</strong>itoring systems needed, are more problematicthan those required for iodized salt (296).5.3.2.4 MilkIodine-enriched milk has been instrumental in the c<strong>on</strong>trol of iodine deficiencyin several countries. However, this has been largely a c<strong>on</strong>sequence of the use ofiodophors by the dairy industry rather than the result of a deliberate additi<strong>on</strong> ofiodine to milk. Iodine-enriched milk has become a major adventitious sourceof iodine in many countries in northern Europe, as well as in the UnitedKingdom (298) and the United States. Use of iodized bread in Tasmania wasdisc<strong>on</strong>tinued when other sources of iodine, notably milk (c<strong>on</strong>sequent to the useof iodophors by the dairy industry), became available.5.3.2.5 Other vehiclesThe feasibility of using sugar as a vehicle for iodine fortificati<strong>on</strong> has beenassessed in pilot studies in Sudan (299), and that of fish sauce in south-east Asiawhere it is a major source of dietary sodium (i.e. salt). Besides fortifying tablesalt (300). Finland fortifies its animal fodders and as a result the iodine c<strong>on</strong>tentof <strong>food</strong>s derived from animal sources has increased.5.3.3 Safety issuesIodine fortificati<strong>on</strong> is generally very safe. Iodine has been added to salt and breadfor more than 50 years <strong>with</strong>out any notable toxic effects (301). At its fifty-thirdmeeting in 1999, the Joint FAO/WHO Expert Committee <strong>on</strong> Food Additivesc<strong>on</strong>cluded that potassium iodate and potassium iodide could c<strong>on</strong>tinue to be usedto fortify salt for the preventi<strong>on</strong> and c<strong>on</strong>trol of iodine deficiency disorders (238).Because the synthesis and release of thyroid horm<strong>on</strong>es is usually well regulated,through mechanisms that enable the body to adjust to a wide range of iodineintakes, intakes of up to 1 mg (1 000 µg) per day are tolerated by most people.Nevertheless, an acute, excessive increase in iodine intake can increase therisk of iodine toxicity in susceptible individuals, that is, those who havehad chr<strong>on</strong>ic iodine deficiency. This c<strong>on</strong>diti<strong>on</strong> is known as iodine-induced122
5. IRON, VITAMIN A AND IODINEhyperthyroidism (IIH) and it is the most comm<strong>on</strong> complicati<strong>on</strong> of iodine prophylaxis.Outbreaks have been associated <strong>with</strong> almost all iodine supplementati<strong>on</strong>programmes (302); it tends to occur in the early phase of programmeimplementati<strong>on</strong> and mainly affects the elderly who have l<strong>on</strong>g-standing thyroidnodules. IIH is, however, usually transitory in nature and its incidence ratereverts to normal levels after 1–10 years of interventi<strong>on</strong>.Outbreaks of IIH, which were subsequently attributed to the suddenintroducti<strong>on</strong> of excessively iodized salt in populati<strong>on</strong>s who had been severelyiodine deficient for very l<strong>on</strong>g periods, have recently been reported from theDemocratic Republic of the C<strong>on</strong>go (303) and Zimbabwe (304). Such reportswould appear to indicate that IIH could occur if salt is excessively iodized (305).If an outbreak of IIH was to occur following the introducti<strong>on</strong> of iodized salt,it would be expected to follow a similar pattern to that observed duringiodine supplementati<strong>on</strong> programmes, that is, manifest early <strong>on</strong> in the history ofthe programme and predominantly am<strong>on</strong>g the elderly. IIH preventi<strong>on</strong> requiresthe m<strong>on</strong>itoring of salt iodizati<strong>on</strong> levels and the iodine status of the populati<strong>on</strong>,coupled <strong>with</strong> proper training of health staff in the identificati<strong>on</strong> and treatmentof IIH (306).Iodine-induced thyroiditis is another c<strong>on</strong>diti<strong>on</strong> that can be aggravated or eveninduced by increasing iodine intakes (307). To date, there have been no largescaleinvestigati<strong>on</strong>s of the impact of iodine interventi<strong>on</strong> programmes <strong>on</strong> iodineinducedthyroiditis.Summary: iodine fortificati<strong>on</strong>■ Universal salt iodizati<strong>on</strong>, that is, the iodizati<strong>on</strong> of all salt for both human and animalc<strong>on</strong>sumpti<strong>on</strong>, is the strategy recommended by WHO to correct iodine deficiency.■ Potassium iodate is preferred to potassium iodide for salt iodizati<strong>on</strong> because it ismore stable.■ The benefits of correcting iodine deficiency far outweigh the potential risks of fortificati<strong>on</strong>.Iodine-induced hyperthroidism and other potential adverse effects can bealmost entirely avoided by adequate and sustained quality assurance and m<strong>on</strong>itoringof iodine fortificati<strong>on</strong>.123
CHAPTER 6Zinc, folate and other B vitamins,vitamin C, vitamin D, calcium, seleniumand fluoride6.1 Zinc6.1.1 Choice of zinc fortificantZinc compounds that are suitable for use as <strong>food</strong> fortificants include the sulfate,chloride, gluc<strong>on</strong>ate, oxide and the stearate. All of these compounds are eitherwhite or colourless, but have varying water solubilities; some have an unpleasanttaste when added to certain <strong>food</strong>s. Although it is <strong>on</strong>ly poorly water soluble,zinc oxide is the cheapest of the zinc fortificants and therefore tends to be thepreferred choice. Recent studies have shown that the absorpti<strong>on</strong> of zinc fromcereal products fortified <strong>with</strong> zinc oxide is as good as that from those fortified<strong>with</strong> the more soluble zinc sulfate (308,309), presumably because the oxide issoluble in gastric acid. However, zinc absorpti<strong>on</strong> from the oxide may be poorin individuals <strong>with</strong> low stomach acid secreti<strong>on</strong>.6.1.2 The bioavailability of zincZinc absorpti<strong>on</strong> from <strong>food</strong> is dependent <strong>on</strong> the amount of zinc c<strong>on</strong>sumed andthe ratio of phytate to zinc in the meal being c<strong>on</strong>sumed. According to recentestimates by the Internati<strong>on</strong>al Zinc Nutriti<strong>on</strong> C<strong>on</strong>sultative Group (IZiNCG),when zinc intake is just adequate to meet the physiological requirements forabsorbed zinc, in adult men about 27% of the zinc c<strong>on</strong>tent is absorbed fromdiets having a phytate:zinc molar ratio of less than 18, which drops to about 19%when the phytate:zinc molar ratio is greater than 18 (i.e. high phytate). The corresp<strong>on</strong>dingzinc absorpti<strong>on</strong> rates for adult women are 35% and 26%, respectively(109). When zinc intake is greater than the critical level needed to meetrequirements, the fracti<strong>on</strong>al absorpti<strong>on</strong> becomes progressively less, although thenet absorpti<strong>on</strong> of zinc increases slightly. In <strong>on</strong>e study involving healthy, wellnourishedadults from the United States, zinc absorpti<strong>on</strong> from the sulfate (orthe oxide) added to a low-phytate bread meal was about 14% (total zinc c<strong>on</strong>tent,3.1–3.7 mg per meal) compared <strong>with</strong> around 6% from the same fortificantsadded to a high-phytate wheat porridge meal (total zinc c<strong>on</strong>tent, 2.7–3.1 mg permeal) (309).124
6. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDE6.1.3 Methods used to increase zinc absorpti<strong>on</strong> from fortificantsIn light of the above findings, and given the similarities to ir<strong>on</strong> (see secti<strong>on</strong> 5.1.2),it is reas<strong>on</strong>able to assume that reducing the phytic acid c<strong>on</strong>tent of <strong>food</strong> willincrease the absorpti<strong>on</strong> of zinc from fortificants, at least in the case of adults(310). Whether the same applies to infants and young children is uncertain. Alower extracti<strong>on</strong> rate will result in a reduced phytate c<strong>on</strong>tent of cereals but alsoa reduced zinc c<strong>on</strong>tent, so the net effect <strong>on</strong> zinc supply tends to be minimal.Alternatively, the phytate c<strong>on</strong>tent can be reduced by activating the phytases thatare naturally present in most phytate-c<strong>on</strong>taining <strong>food</strong>s (through germinati<strong>on</strong>,fermentati<strong>on</strong> and/or soaking) or by adding microbial or fungal phytases. Includingsources of animal protein in the diet has also been shown to be an effectiveway of improving zinc absorpti<strong>on</strong> from high-phytate diets (93).Absorpti<strong>on</strong> enhancers equivalent to ascorbic acid for ir<strong>on</strong>, do not exist forzinc. However, according to the results of <strong>on</strong>e study c<strong>on</strong>ducted in adult women,the additi<strong>on</strong> of NaFeEDTA as a fortificant can increase zinc absorpti<strong>on</strong> fromthe diet, in this case from about 20% to 35%; 1% of the additi<strong>on</strong>al amount ofzinc absorbed was excreted in the urine (311). This finding has yet to be c<strong>on</strong>firmedin other studies. However, if, as reports suggest, the additi<strong>on</strong> ofNa 2 EDTA or NaFeEDTA to cereal flours inhibits the acti<strong>on</strong> of yeast during thebread-making process, these compounds would be of limited use, at least incereal flours.6.1.4 Experience <strong>with</strong> zinc fortificati<strong>on</strong> of specific <strong>food</strong>sHitherto, fortificati<strong>on</strong> <strong>with</strong> zinc has been fairly limited, and is generally c<strong>on</strong>finedto infant formula milks (<strong>with</strong> zinc sulfate), complementary <strong>food</strong>s and ready-toeatbreakfast cereals (in the United States). In Ind<strong>on</strong>esia it is mandatory to addzinc to wheat noodles. More recently, several Latin American countries haveexpressed some interest in fortifying cereal flours <strong>with</strong> zinc.Several studies have dem<strong>on</strong>strated the benefits of zinc supplementati<strong>on</strong> <strong>on</strong>the growth rate of children (see secti<strong>on</strong> 4.1.3). However, very few trials haveassessed the efficacy or effectiveness of zinc fortificati<strong>on</strong>. Although the additi<strong>on</strong>of zinc oxide to breakfast cereals increased plasma zinc c<strong>on</strong>centrati<strong>on</strong>s in preschool-agedchildren in the United States, there was no evidence of c<strong>on</strong>comitantincreases in growth rates or in <strong>food</strong> intake (312). However, in Turkey, zincfortificati<strong>on</strong> of bread did increase the growth rates of schoolchildren who initiallyhad low plasma zinc (313).Little is known about the effects of added zinc <strong>on</strong> the sensory properties of<strong>food</strong>s. The fortificati<strong>on</strong> of wheat flour <strong>with</strong> relatively high levels of zinc (as zincacetate) did not affect the baking or organoleptic properties of the bread dough(313). Likewise, the additi<strong>on</strong> of 60 or 100 mg zinc/kg wheat flour (as zinc sulfateor zinc oxide) did not change the acceptability of bread (314). Encapsulati<strong>on</strong> of125
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSzinc compounds is possible but has not been c<strong>on</strong>sidered to date. This would,however, be a c<strong>on</strong>venient way to mask the unpleasant taste of some zinccompounds.6.2 Folate and other B vitaminsThe B-complex vitamins are c<strong>on</strong>sidered as a group in this chapter, as not <strong>on</strong>lydo they share some similar characteristics when used as <strong>food</strong> fortificants butthey also tend to be added to the same <strong>food</strong>s. Members of the group of Bvitamins covered here include folate/folic acid (vitamin B 9 ), thiamine (vitaminB 1 ), riboflavin (vitamin B 2 ), niacin, pyridoxine (vitamin B 6 ) and vitamin B 12(cobalamin).6.2.1 Choice of vitamin B fortificantsThe characteristics of the vitamin B compounds that are suitable for addingto <strong>food</strong>s are summarized in Table 6.1. In general, the B vitamins are relativelystable, <strong>with</strong> thiamine being the most labile to heat. Synthetic folate,i.e. folic acid (in the form of pteroyl m<strong>on</strong>oglutamic acid) is moderatelyheat stable (315), but is susceptible to the effects of oxidizing and reducingagents (316).Some fortificant loss is inevitable, the degree of loss being dependent <strong>on</strong>factors such as the temperature used during <strong>food</strong> processing or preparati<strong>on</strong>, themoisture c<strong>on</strong>tent, extrusi<strong>on</strong> temperatures and pressures, the presence of othermicr<strong>on</strong>utrients (in the premix and in the fortified <strong>food</strong>), the nature of the packaging,and the anticipated shelf-life of the fortified product. Vitamin recoveriesin bread made from fortified flour range from about 70% to 95% for niacin, andfrom 75% to 90% for thiamine and pyridoxine. About 70% of any added thiamine,pyridoxine and niacin is retained when enriched flour is used to preparepasta, even after drying and cooking. On this basis, and assuming that any addedB vitamins are 100% absorbed, in flour an overage of approximately 20–30% isthus usually sufficient to provide the desired amount in <strong>food</strong> products such asbreads and cereals.Folic acid has a light yellow colour, which does not carry over to fortified<strong>food</strong>s because it is added at such low levels, typically between 1.5 and 2.4 ppm.There is some loss of the vitamin <strong>on</strong> exposure to light, and during cooking andbaking. The biggest losses tend to occur from biscuits and pasta, but even theseare probably no more than 20%. As folic acid c<strong>on</strong>centrati<strong>on</strong>s in <strong>food</strong>s are difficultto measure, reported levels in fortified flour and baked products are oftensubject to c<strong>on</strong>siderable assay error.126
6. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDETABLE 6.1Vitamin B fortificants: physical characteristics and stabilityVitamin Fortificant Physical StabilitycompoundcharacteristicsThiamine Thiamine More soluble in Both salts are stable to oxygen(B 1 ) hydrochloride water than the in the absence of light andm<strong>on</strong><strong>on</strong>itrate form moisture but are unstable inWhite or almost neutral or alkaline soluti<strong>on</strong>swhiteand in the presence ofsulfites.Thiamine White or almost Losses during leavening andm<strong>on</strong><strong>on</strong>itrate white baking are estimated to be15–20%.Available in a coated form.The m<strong>on</strong><strong>on</strong>itrate is preferred fordry products.Riboflavin Riboflavin Relatively water Very unstable in light.(B 2 ) insoluble Rapid loss from milk <strong>on</strong>Yellowexposure to light but stablein white bread.Sodium salt of Soluble in waterriboflavin 5′- YellowphosphateNiacin Niacin (nicotinic Soluble in alkali, Very stable to oxygen, heat andacid) sparingly soluble light, both in the dry statein waterand in aqueous soluti<strong>on</strong>.WhiteNiacinamide Water soluble(nicotinamide) WhitePyridoxine Pyridoxine Water soluble Stable in oxygen and heat, but(B 6 ) hydrochloride White or almost relatively sensitive to UVwhitelight.Available in a coated form.Folic acid Pteroyl Sparingly soluble in Moderately stable to heat.(B 9 ) m<strong>on</strong>oglutamic water, soluble in Stable in soluti<strong>on</strong> at neutral pHacid dilute acid and but increasingly unstable atalkalihigher or lower pH.Yellow-orange Unstable in UV light.Vitamin B 12 Cyanocobalamin Pure vitamin B 12 is Relatively stable to oxygen and(cobalamin) sparingly soluble heat in neutral and acidin water; the Soluti<strong>on</strong>, but unstable indiluted formsalkali and str<strong>on</strong>g acids, inare howeverstr<strong>on</strong>g light, and in alkalinecompletely soluble soluti<strong>on</strong>s at >100°C.Dark red, oftensupplied diluted<strong>on</strong> a carrier (0.1%)127
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS6.2.2 Experience <strong>with</strong> vitamin B fortificati<strong>on</strong> of specific <strong>food</strong>sThere is a l<strong>on</strong>g history of experience of adding B vitamins to cereals (includingwheat and maize flours) and rice grains, in both industrialized and developingcountries.The benefits of restorati<strong>on</strong> of thiamine, riboflavin and niacin in cerealsand flours, 65–80% of which are removed by milling, have l<strong>on</strong>g been recognized.Indeed, the enrichment of flours and cereals has made, and c<strong>on</strong>tinues to make,a major c<strong>on</strong>tributi<strong>on</strong> to meeting the recommended intake of these vitamins evenin the industrialized countries (317). The amount of niacin added to wheat flourtypically ranges from 15 to 70 mg/kg (178); thiamine (vitamin B 1 ) additi<strong>on</strong> levelsrange from 1.5 to 11 mg/kg, and those for vitamin B 12 , from 1.3 to 4 mg/kg (318).About 75% of the folate in whole wheat is also lost during milling, but folicacid has been included in cereal fortificati<strong>on</strong> programmes <strong>on</strong>ly relatively recently.In 1998, it became mandatory to fortify grain products <strong>with</strong> folic acid in theUnited States, the rati<strong>on</strong>ale being that it would lower the prevalence of neuraltube defect births.The required fortificati<strong>on</strong> level is 154 µg/100 g flour (Mandate21 CFR 137.165). According to <strong>on</strong>e assessment the impact of this measure hasbeen a 26% reducti<strong>on</strong> in the incidence of neural tube defects (48). Mandatoryfolate acid fortificati<strong>on</strong> has also quite rapidly lowered the prevalence of lowplasma folate c<strong>on</strong>centrati<strong>on</strong>s in adults from around 22% to almost zero, andreduced the prevalence of elevated plasma homocysteine by about 50% (49). Inadditi<strong>on</strong> to the United States, some 30 countries now add folic acid to flour,including Canada (150 µg/100 g), Chile (220µg/100 g wheat flour), Costa Rica(180 µg/100 g), Dominican Republic (180µg/100 g), El Salvador (180µg/100 g),Guatemala (180 µg/100g), H<strong>on</strong>duras (180 µg/100g), Ind<strong>on</strong>esia (200µg/100 gwheat flour), Mexico (200µg/100 g wheat flour), Nicaragua (180µg/100 g) andPanama (180 µg/100 g) (318).The B-complex vitamins are added directly to flour as single nutrients or asa premix (which usually also c<strong>on</strong>tains ir<strong>on</strong>), or they are diluted <strong>with</strong> a smallamount of flour at the mill before being added to the bulk. In the case of readyto-eatbreakfast cereals, the B vitamins can either be added to the dry mix priorto extrusi<strong>on</strong> or other processes, or a vitamin soluti<strong>on</strong> or suspensi<strong>on</strong> can besprayed <strong>on</strong>to the cereals after they have been toasted. Riboflavin has a str<strong>on</strong>gyellow colour and slightly bitter taste, but at the levels that are typically addedto white flour any colour or taste problems are likely to be minimal. Coatedforms of the water-soluble vitamins, such as thiamine and vitamin B 6 , are availableif off-flavours or other problems arise (Table 6.1).6.2.3 Safety c<strong>on</strong>cerns6.2.3.1 Thiamine, riboflavin and vitamin B 6As toxicity is not a problem, the United States Food and Nutriti<strong>on</strong> Board hasnot defined upper intake limits (ULs) for thiamine and riboflavin. In the case128
6. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEof vitamin B 6 , sensory neuropathy has been linked to high intakes of supplementsbut according to the findings of the United States Food and Nutriti<strong>on</strong>Board, “No adverse effects associated <strong>with</strong> vitamin B 6 from <strong>food</strong> have beenreported. This does not mean that there is no potential for adverse effects resultingfrom high intakes. Because data <strong>on</strong> the adverse effects of vitamin B 6 arelimited, cauti<strong>on</strong> may be warranted”. A UL of 100 mg for adults and 30–40 mgfor children has thus been set (128).These levels are very unlikely to be obtainedfrom fortified <strong>food</strong>s.6.2.3.2 Niacin (nicotinic acid and niacinamide)Vasodilati<strong>on</strong> or flushing (i.e. a burning or itching sensati<strong>on</strong> in the face, arms andchest) has been observed as a first adverse effect in patients given high doses ofnicotinic acid for the treatment of hyperlipidemia. Based <strong>on</strong> such evidence, theUnited States Food and Nutriti<strong>on</strong> Board has defined a UL of 35 mg/day fornicotinic acid (128). Intakes of niacinamide have, however, not been associated<strong>with</strong> flushing effects.Bearing in mind the different characteristics of the two forms of niacin, theScientific Committee for Food in the European Uni<strong>on</strong> has proposed a UL fornicotinic acid of 10 mg/day and a separate, much higher, UL for niacinamide of900 mg/day (319). The latter thus poses no safety limitati<strong>on</strong>s in comm<strong>on</strong> <strong>food</strong>fortificati<strong>on</strong> practice.6.2.3.3 Folic acid fortificantsThe c<strong>on</strong>sumpti<strong>on</strong> of folic acid in amounts normally found in fortified <strong>food</strong>s hasnot been associated <strong>with</strong> adverse health effects. However, there has been somec<strong>on</strong>cern that high folic acid intakes could mask or exacerbate neurological problems,such as pernicious anaemia, in people <strong>with</strong> low intakes of vitamin B 12(128). This has led to a reluctance to fortify <strong>with</strong> folic acid in some countries.This c<strong>on</strong>cern is particularly pertinent to those individuals who derive folic acidfrom both supplements and a range of fortified <strong>food</strong>s, as it is the case in manyindustrialized countries. In this situati<strong>on</strong>, some people may exceed the UL forfolic acid, which has been set at 1 mg/day (128)(129 old 110). An obvious soluti<strong>on</strong>to this potential problem is to fortify <strong>food</strong>s <strong>with</strong> both vitamin B 12 and folicacid.To avoid any possible risk of adverse effects, folic acid fortificati<strong>on</strong> programmesshould be designed so as to limit regular daily intakes to a maximumof 1 mg. In additi<strong>on</strong>, measures which require folic acid-c<strong>on</strong>taining supplementsand fortified <strong>food</strong>s to also c<strong>on</strong>tain vitamin B 12 could be c<strong>on</strong>sidered, especiallyin the case of products c<strong>on</strong>sumed by older citizens who are at greater risk ofvitamin B 12 deficiency and its associated c<strong>on</strong>diti<strong>on</strong>s, in particular, perniciousanaemia.129
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS6.3 Vitamin C (ascorbic acid)6.3.1 Choice of vitamin C fortificantAscorbic acid and ascorbyl palmitate are often added to oils, fats, soft drinks andvarious other <strong>food</strong>s as a way of improving the stability of other added micr<strong>on</strong>utrients(e.g. vitamin A) or as an ir<strong>on</strong> absorpti<strong>on</strong> enhancer (see secti<strong>on</strong> 5.1.2.1).However, ascorbic acid is itself relatively unstable in the presence of oxygen,metals, humidity and/or high temperatures. To retain vitamin C integrity (especiallyduring storage), <strong>food</strong>s must therefore be appropriately packaged, or theascorbic acid encapsulated.6.3.2 Experience <strong>with</strong> vitamin C fortificati<strong>on</strong> of specific <strong>food</strong>sAs a general rule, <strong>food</strong>s that are not cooked are better vehicles for vitamin Cfortificati<strong>on</strong>. Blended <strong>food</strong>s, such as those used for feeding programmes in emergencysituati<strong>on</strong>s, were often fortified <strong>with</strong> vitamin C as this was believed to bethe most efficient way of delivering this nutrient to populati<strong>on</strong>s likely to be deficient.However, a trial <strong>with</strong> PL-480 cereals found that although almost all of theencapsulated fortificant ascorbic acid was retained during transit from theUnited States to Africa, it was rapidly destroyed when the cereal product wascooked for 10 minutes (270). On the other hand, the additi<strong>on</strong> of vitamin C tocommercially processed <strong>food</strong>s such as dry milk, infant formulas, cereal-basedcomplementary <strong>food</strong>s, chocolate drink powders and beverages has been foundto be successful in increasing intakes of this nutrient. As sugar helps to protectthe ascorbic acid in soft drinks, sugar has been proposed as a possible vehiclefor the vitamin (184).6.4 Vitamin D6.4.1 Choice of vitamin D fortificantEither vitamin D 2 (ergocalciferol) or D 3 (cholecalciferol) can be added to <strong>food</strong>s.The two forms have similar biological activities and both are very sensitive tooxygen and moisture, and both interact <strong>with</strong> minerals. A dry stabilized form ofvitamin D, which c<strong>on</strong>tains an antioxidant (usually tocopherol) that protectsactivity even in the presence of minerals, is generally used for most commercialapplicati<strong>on</strong>s.6.4.2 Experience <strong>with</strong> vitamin D fortificati<strong>on</strong> of specific <strong>food</strong>sMilk and other dairy products, including dried milk powder and evaporatedmilk, are often fortified <strong>with</strong> vitamin D. Many countries also fortify margarines<strong>with</strong> this vitamin.Low exposure to sunlight is a risk factor for vitamin D deficiency and can bea problem am<strong>on</strong>g those who live in the more northerly or southerly latitudes130
6. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEwhere UV radiati<strong>on</strong> levels are lower during the winter m<strong>on</strong>ths, and am<strong>on</strong>gwomen who, for cultural reas<strong>on</strong>s, spend a large proporti<strong>on</strong> of their time indoorsor covered <strong>with</strong> clothing. In such situati<strong>on</strong>s, vitamin D fortificati<strong>on</strong> of milk andmargarine have been found to be useful strategies for increasing intakes; the goalis to supply up to 200 IU/day in the total diet.6.5 CalciumCompared <strong>with</strong> other micr<strong>on</strong>utrients, calcium is required in relatively largeamounts. A heightened awareness of the need to increase intakes of calcium forosteoporosis preventi<strong>on</strong> has meant that calcium fortificati<strong>on</strong> has attracted a gooddeal of interest in recent years.6.5.1 Choice of calcium fortificantsCalcium salts suitable for use as <strong>food</strong> fortificants are listed in Table 6.2. Bioavailableforms recommended for the fortificati<strong>on</strong> of infant formulas and complementary<strong>food</strong>s include the carb<strong>on</strong>ate (it can liberate CO 2 in acid systems), thechloride, the citrate and the citrate malate, the gluc<strong>on</strong>ate, the glycerophosphate,the lactate, the m<strong>on</strong>o-, di- and tribasic phosphates, the orthophosphate, thehydroxide and the oxide (320). All of these salts are either white or colourless.Most are bland although the citrate has a tart flavour, the hydroxide is slightlybitter, and high c<strong>on</strong>centrati<strong>on</strong>s of the chloride and the lactate can be unpleasant.The cost of calcium carb<strong>on</strong>ate is very low, usually less than that of flour.As the daily amount of calcium required is several thousand times higher thanthat of most other micr<strong>on</strong>utrients, it tends to be added separately (as opposedto part of a premix). The calcium c<strong>on</strong>tent of commercially available salts rangesfrom 9% (the gluc<strong>on</strong>ate) to 71% (the oxide) (Table 6.2). Salts <strong>with</strong> lower c<strong>on</strong>centrati<strong>on</strong>swill have to be added in larger amounts, a factor that may affect thefinal choice of fortificant.There is little reas<strong>on</strong> to believe that low solubility is a major c<strong>on</strong>straint to thebioavailability of fortificant calcium. In general, absorpti<strong>on</strong> of added calcium issimilar to that naturally present in <strong>food</strong>s, which ranges from about 10% to 30%.However, high levels of calcium inhibit the absorpti<strong>on</strong> of ir<strong>on</strong> from <strong>food</strong>s andso this too is something that needs to be taken into c<strong>on</strong>siderati<strong>on</strong> when decidinghow much calcium to add. The co-additi<strong>on</strong> of ascorbic acid can help overcomethe inhibitory effect of calcium <strong>on</strong> ir<strong>on</strong> absorpti<strong>on</strong>.6.5.2 Experience <strong>with</strong> calcium fortificati<strong>on</strong>Wheat flour was first fortified <strong>with</strong> calcium in the United Kingdom in 1943 inorder to restore the calcium lost during milling. Today, it is compulsory to add940–1560 mg calcium carb<strong>on</strong>ate/kg to white and brown (but not wholegrain)131
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 6.2Calcium fortificants: physical characteristicsCompound Calcium Colour Taste Odour Solubilityc<strong>on</strong>tent(mmol/l)(%)Carb<strong>on</strong>ate 40 Colourless Soapy, lem<strong>on</strong>y Odourless 0.153Chloride 36 Colourless Salty, bitter – 6 712Sulfate 29 Varies – – 15.3Hydroxyapatite 40 – – – 0.08Calcium phosphate 30 White Sandy, bland – 1.84dibasicCalcium phosphate 17 Colourless Sandy, bland – 71.4m<strong>on</strong>obasicCalcium phosphate 38 White Sandy, bland Odourless 0.064tribasicCalcium 31 Colourless – – InsolublepyrophosphateGlycerophosphate 19 White Almost Odourless 95.2tastelessAcetate 25 Colourless – – 2 364Lactate 13 White Neutral Almost 0.13odourlessCitrate 24 Colourless Tart, clean Odourless 1.49Citrate malate 23 Colourless – – 80.0Gluc<strong>on</strong>ate 9 White Bland Odourless 73.6Hydroxide 54 Colourless Slightly bitter Odourless 25.0Oxide 71 Colourless – – 23.3Source: adapted from reference (320).flours milled in the United Kingdom. In the United States, the additi<strong>on</strong> ofcalcium to flour has been opti<strong>on</strong>al since the early 1940s. Calcium sulfate, carb<strong>on</strong>ate,chloride, phosphate, acetate or lactate are all suitable for fortificati<strong>on</strong> ofwheat flours, but the oxide and hydroxide may require alterati<strong>on</strong>s in the pH ofthe dough for successful bread-making (321).The range of <strong>food</strong>s that are fortified <strong>with</strong> calcium has steadily grown over theyears as it became increasingly clear that intakes were low in many populati<strong>on</strong>s.The more soluble calcium salts, such as the citrate malate or the gluc<strong>on</strong>ate, aregenerally used to fortify juices and other beverages. Tribasic calcium phosphate,and sometimes calcium carb<strong>on</strong>ate or lactate, is used to fortify milk, to whichgums (e.g. carrageenan, guar gum) must also be added to prevent the calciumsalt from sedimenting.Yoghurt and cottage cheese can also be fortified <strong>with</strong> thesecalcium compounds. In industrialized nati<strong>on</strong>s and in some Asian countries, soyabeverages are marketed as a replacement for cow’s milk in which case these tooshould be fortified <strong>with</strong> calcium. Stabilizers such as sodium hexametaphosphate132
6. ZINC, B VITAMINS, VITAMINS C AND D, CALCIUM, SELENIUM AND FLUORIDEor potassium citrate can improve the quality of soya beverages fortified <strong>with</strong>calcium gluc<strong>on</strong>ate or lactogluc<strong>on</strong>ate.The additi<strong>on</strong> of calcium salts to some <strong>food</strong>s can cause undesirable changesin colour, texture and stability by increasing the cross-linking of proteins, pectinsand gums. Calcium fortificants can also darken the colour of chocolatebeverages.6.6. Selenium6.6.1 Choice of fortificantFor <strong>food</strong> fortificati<strong>on</strong> purposes, the sodium salts are generally c<strong>on</strong>sidered to bethe most suitable source of selenium. The selenite is a white, water-soluble compound,from which absorpti<strong>on</strong> is about 50%. It is readily reduced to unabsorbableelemental selenium by reducing agents, such as ascorbic acid and sulfurdioxide. Sodium selenate is colourless, and is less soluble in water and morestable than the selenite, especially in the presence of copper and ir<strong>on</strong>. It has thebetter absorpti<strong>on</strong> (nearly 100% from the fortificant al<strong>on</strong>e or 50–80% depending<strong>on</strong> the <strong>food</strong> vehicle to which it has been added), and also increases the activityof the enzyme, glutathi<strong>on</strong>e peroxidase, more effectively. When tested inmilk-based infant formulas, more selenium was absorbed from the selenate (97%versus 73%), but as more selenium was excreted in the urine <strong>with</strong> the selenate(36% versus 10%), the net retenti<strong>on</strong> of selenium appears to be similar regardlessof which chemical form is used (322). The relative retenti<strong>on</strong> of seleniumfrom other fortified <strong>food</strong>s, including salt, has not been investigated. Organicforms of selenium, such as selenomethi<strong>on</strong>ine, are absorbed as well as the selenate,but remain l<strong>on</strong>ger in the body and thus theoretically pose a higher risk oftoxicity. They have not been widely used for <strong>food</strong> fortificati<strong>on</strong> for this reas<strong>on</strong>.6.6.2 Experience <strong>with</strong> selenium fortificati<strong>on</strong> of selected <strong>food</strong>sIn regi<strong>on</strong>s of China where selenium deficiency is endemic, salt has been fortified<strong>with</strong> sodium selenite (15 mg/kg) since 1983.This measure increased averagedaily selenium intakes from 11µg to 80 µg and has effectively reduced the prevalenceof Keshan disease (see also secti<strong>on</strong> 4.8.3).Sodium selenate is currently used to fortify a range of <strong>food</strong>s in various partsof the world. In Finland, for example, sodium selenate is added to fertilizersapplied in areas having low soil selenium; measurable increases in the seleniumc<strong>on</strong>tent of milk, meat and cereals grown <strong>on</strong> these soils were observed <strong>with</strong>in 6m<strong>on</strong>ths (217). Sodium selenate is an ingredient in some sports drinks (around10µg/l) and in the United States is used to fortify infant <strong>food</strong>s. Until 1985, breadsupplied about half of the selenium intake for the United Kingdom populati<strong>on</strong>,but after 1985, when European wheat was replaced by Canadian wheat thisdropped to about 20%.133
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS6.7 Fluoride6.7.1 Choice of fortificantThere are a number of ways in which fluoride intakes can be increased: fluoridecan be added to water supplies at the point of supply or added to toothpaste.Hexa-fluoro-silicate acid (HUSIAC) is the most comm<strong>on</strong>ly used fluoridecompound for large-scale water fortificati<strong>on</strong>. It is added as a c<strong>on</strong>centratedaqueous soluti<strong>on</strong>. The fluoridati<strong>on</strong> of salt and the enrichment of milk <strong>with</strong> fluorideare alternative opti<strong>on</strong>s that have been used in some parts of the world.6.7.2 Experience of fluoridati<strong>on</strong>The introducti<strong>on</strong> of a salt fluoridati<strong>on</strong> programme in Jamaica was associated<strong>with</strong> a large reducti<strong>on</strong> in dental decay in children, when assessed after 7 years(323). However, a smaller trial in Hungary indicated that residence during earlyinfancy in an area where salt was fluoridated was not associated <strong>with</strong> a reducedrisk of later caries (324). In Costa Rica, a nati<strong>on</strong>al fluoride salt fortificati<strong>on</strong> programme,requiring the additi<strong>on</strong> of 225–275 mg fluoride/kg salt, became mandatoryin 1989. There then followed a very substantial and progressive reducti<strong>on</strong>in tooth decay, and in 1999, based <strong>on</strong> measurements of urinary fluorine excreti<strong>on</strong>rates, the level of fluoride in salt was lowered to 175–225 mg/kg (325).However, it is possible that other sources of fluoride (i.e. toothpaste) may havec<strong>on</strong>tributed to the observed reducti<strong>on</strong> in the prevalence of tooth decay inCosta Rica.Where it is impractical or unacceptable to fluoridate water or salt, the additi<strong>on</strong>of fluoride to milk is an alternative approach for preventing dental caries.Generally speaking, the level of fluoridati<strong>on</strong> is best governed by the usual volumeof milk c<strong>on</strong>sumed by young children. <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for fluoride fortificati<strong>on</strong> of milkand milk products are available elsewhere (326).A recent evaluati<strong>on</strong> of the feasibility of adding fluoride to school milk in theUnited Kingdom c<strong>on</strong>cluded that fortificati<strong>on</strong> was both feasible and desirable(327). In rural Chile, preschool-aged children received 0.25–0.75 mg fluorideper day in fortified, powdered milk for a period of 4 years. The rate of decayed,missing and filled teeth declined substantially compared <strong>with</strong> a c<strong>on</strong>trol community,and the percentage of children who remained caries-free doubled (328).Favourable results have also been reported from Beijing, in children who c<strong>on</strong>sumed0.5 mg fluoride in milk each day at kindergarten and 0.6 mg fluoride inmilk at home <strong>on</strong> weekend days (329). Similarly, in Scotland schoolchildren whoc<strong>on</strong>sumed 1.5 mg fluoride daily in 200 ml milk had a significantly lower prevalenceof caries than a c<strong>on</strong>trol group after 5 years (330). However, these resultswere not replicated in a more recent study c<strong>on</strong>ducted in another regi<strong>on</strong> of theUnited Kingdom (331).134
PA RT I VImplementing effectiveand sustainable <strong>food</strong>fortificati<strong>on</strong> programmes
Introducti<strong>on</strong>As the preceding chapters have dem<strong>on</strong>strated, <strong>food</strong> fortificati<strong>on</strong> has a l<strong>on</strong>ghistory of successful practice. Notable successes have been in achieved in thecase of the iodizati<strong>on</strong> of salt, the fortificati<strong>on</strong> of flour <strong>with</strong> various B vitamins,and the fortificati<strong>on</strong> of margarines <strong>with</strong> vitamin A. It would, however, be somethingof an overstatement to say that these past successes have been the resultof formal, scientifically-rigorous evaluati<strong>on</strong>s of the nutriti<strong>on</strong>al status and needsof the target populati<strong>on</strong>. In many cases, decisi<strong>on</strong>s about how much fortificantto add to a chosen <strong>food</strong> vehicle were based <strong>on</strong> what was known to be technicallypossible at the time, and governed by budgetary limits.With a view to putting fortificati<strong>on</strong> programme planning <strong>on</strong> a sounderfooting, this secti<strong>on</strong> of the present <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> sets out a systematic and methodologicalapproach to designing and planning a <strong>food</strong> fortificati<strong>on</strong> programme.The key elements are as follows:— defining and setting nutriti<strong>on</strong>al goals (i.e. framing decisi<strong>on</strong>s about howmuch micr<strong>on</strong>utrient(s) to add to which <strong>food</strong>s);— programme m<strong>on</strong>itoring and evaluati<strong>on</strong> (i.e. establishing procedures whichcheck that fortified <strong>food</strong>s c<strong>on</strong>tain the intended amount of micr<strong>on</strong>utrient(s)and that they are being c<strong>on</strong>sumed by the target populati<strong>on</strong> in adequateamounts);— communicating and marketing fortificati<strong>on</strong> programmes (i.e. informingthe target populati<strong>on</strong> about the benefits of fortificati<strong>on</strong> so that they choseto c<strong>on</strong>sume fortified <strong>food</strong>s).In order to be able to correct a micr<strong>on</strong>utrient deficiency in a populati<strong>on</strong>, whichis after all the ultimate goal of any fortificati<strong>on</strong> programme, it is necessary tofirst ascertain the extent of the deficiency and then the increase in intake that isneeded to satisfy the daily requirement for that micr<strong>on</strong>utrient. Chapter 7explains the applicati<strong>on</strong> of the Estimated Average Requirement (EAR) cut-pointmethod to the problem of calculating the level of micr<strong>on</strong>utrient additi<strong>on</strong>s thatis needed to bring the prevalence of low intakes am<strong>on</strong>g a target populati<strong>on</strong> toan acceptably small level. This is the WHO recommended method and is applicableto all micr<strong>on</strong>utrients covered in these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>, <strong>with</strong> the excepti<strong>on</strong> of137
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSir<strong>on</strong> (for which an alternative methodology is described). The informati<strong>on</strong> needsfor such computati<strong>on</strong>s, e.g. data <strong>on</strong> <strong>food</strong> and nutrient intake distributi<strong>on</strong>s, arealso outlined. Having estimated the ideal level of micr<strong>on</strong>utrient additi<strong>on</strong> requiredto achieve a given nutriti<strong>on</strong>al goal, programme planners are then advised to c<strong>on</strong>siderwhether this level of fortificati<strong>on</strong> is feasible given current technology andany safety or cost c<strong>on</strong>straints that may be operating, or whether, additi<strong>on</strong>al measures(e.g. supplementati<strong>on</strong>), may be a better way of reaching nutriti<strong>on</strong>al targets,at least for some populati<strong>on</strong> subgroups. Technological, cost and safety limits arethus defined and a series of examples are provided to show how these can beused to shape the final decisi<strong>on</strong> about fortificati<strong>on</strong> levels appropriate to a givensituati<strong>on</strong>.The primary objective of m<strong>on</strong>itoring and evaluati<strong>on</strong> activities is to ascertainwhether or not a fortificati<strong>on</strong> programme is achieving its nutriti<strong>on</strong>al goals <strong>on</strong>ceit has been implemented. These are critical to the success of any fortificati<strong>on</strong>programme and should be viewed as an integral part of overall programmedesign. M<strong>on</strong>itoring and evaluati<strong>on</strong> activities take place at a number of levels.The main purpose of m<strong>on</strong>itoring is to track the operati<strong>on</strong>al performance (orimplementati<strong>on</strong> efficiency) of a programme. Only after m<strong>on</strong>itoring has establishedthat a fortified product of the desired quality is available and accessibleto the target populati<strong>on</strong> in adequate amounts, can the impact of the interventi<strong>on</strong>be evaluated. To date, relatively few fortificati<strong>on</strong> programmes have beenproperly evaluated, partly because impact evaluati<strong>on</strong> is widely perceived to beboth a complex and costly exercise. The methodologies outlined in Chapter 8aim to demystify the process of evaluating the impact of fortificati<strong>on</strong>programmes. Chapter 9 explores the potential usefulness of the applicati<strong>on</strong>of cost-effectiveness and cost–benefit analysis techniques to <strong>food</strong> fortificati<strong>on</strong>interventi<strong>on</strong>s, something that is also in its infancy. The examples given clearlydem<strong>on</strong>strate that fortificati<strong>on</strong> has the potential to be a particularly cost-effectivesoluti<strong>on</strong> to the problem of micr<strong>on</strong>utrient malnutriti<strong>on</strong> in many settings.In order to ensure that fortified <strong>food</strong>s are c<strong>on</strong>sumed in adequate amounts bythose who require them most, all fortificati<strong>on</strong> programmes will need to be supportedby the right mix of educati<strong>on</strong>al and social marketing activities. Like m<strong>on</strong>itoringand evaluati<strong>on</strong>, this third key element should also be thought about atthe design and planning stages of a fortificati<strong>on</strong> programme. Chapter 10 outlinesthe communicati<strong>on</strong> needs of all the various parties involved in the runningof fortificati<strong>on</strong> programmes, not just the c<strong>on</strong>sumer, and provides guidance <strong>on</strong>how messages might be framed to best meet these needs. An understanding ofthe regulatory envir<strong>on</strong>ment is also essential, and therefore these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> c<strong>on</strong>clude<strong>with</strong> an overview of the mechanisms for regulating fortificati<strong>on</strong> throughnati<strong>on</strong>al <strong>food</strong> laws. Reference to the internati<strong>on</strong>al c<strong>on</strong>text is made whereverrelevant.138
CHAPTER 7Defining and setting programme goals7.1 Informati<strong>on</strong> needsIn order to be able to design a successful fortificati<strong>on</strong> programme that achievesits nutriti<strong>on</strong>al objectives, it is necessary to have first gathered some backgroundinformati<strong>on</strong> and nutriti<strong>on</strong>al data, in particular:• biochemical/chemical data <strong>on</strong> nutriti<strong>on</strong>al status (i.e. data <strong>on</strong> the scale andseverity of specific nutrient deficiencies in different populati<strong>on</strong> groups; seePart II);• data <strong>on</strong> dietary patterns (i.e. the compositi<strong>on</strong> of the usual diet);• detailed informati<strong>on</strong> <strong>on</strong> dietary intakes of micr<strong>on</strong>utrients of interest (i.e. thedistributi<strong>on</strong> of usual intakes of specific micr<strong>on</strong>utrients in a populati<strong>on</strong>).This informati<strong>on</strong> is required to c<strong>on</strong>firm the need and provide a rati<strong>on</strong>ale for afortificati<strong>on</strong> programme. Having established the need for an interventi<strong>on</strong>, thesame informati<strong>on</strong> and data can then be used to identify and prioritize the targetpopulati<strong>on</strong> groups, decide which micr<strong>on</strong>utrients (and in what amounts) shouldbe added to which <strong>food</strong>s, and to identify and understand any c<strong>on</strong>straints (e.g.safety, cost, technological) that may impact <strong>on</strong> the amounts of nutrients that canbe added to given <strong>food</strong>s. Specific data needs are outlined in greater detail below,whereas related issues of a more general nature, but equally important <strong>with</strong>regards to the planning stages of fortificati<strong>on</strong> programmes, are reviewed inBox 7.1.7.1.1 Biochemical and clinical evidence of specific micr<strong>on</strong>utrientdeficienciesPart II of these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> describes how it is possible to classify the severity ofa public health problem caused by specific micr<strong>on</strong>utrient deficiencies usingvarious biochemical and clinical indicators and criteria. Where clinical or biochemicaldata indicate a high prevalence of deficiency of a specific nutrient, thisis usually regarded as being good evidence that the diet is not supplying enoughof that particular micr<strong>on</strong>utrient and that fortificati<strong>on</strong> is warranted. The moresevere and widespread the deficiency, the greater the need for interventi<strong>on</strong>.139
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSBOX 7.1Planning and designing a fort i ficati<strong>on</strong> programme: pre l i m i n a ryc<strong>on</strong>siderati<strong>on</strong>s■ The decisi<strong>on</strong> to implement a micr<strong>on</strong>utrient fortificati<strong>on</strong> programme requiresdocumented evidence that the micr<strong>on</strong>utrient c<strong>on</strong>tent of the diet is insufficientor that fortificati<strong>on</strong> will produce a health benefit. The objective is to lower theprevalence of micr<strong>on</strong>utrient deficiencies in the populati<strong>on</strong> and to optimizehealth.■ In some situati<strong>on</strong>s, an insufficient intake of micr<strong>on</strong>utrients is not the <strong>on</strong>ly riskfactor for micr<strong>on</strong>utrient deficiency. Other factors can play a substantial role,including, for example, the presence of infecti<strong>on</strong>s and parasites (which,am<strong>on</strong>g other things, can c<strong>on</strong>tribute to high rates of anaemia). In these situati<strong>on</strong>s,it is important to determine whether fortificati<strong>on</strong> is a cost-effectivestrategy compared <strong>with</strong> other interventi<strong>on</strong>s (e.g. the c<strong>on</strong>trol of infecti<strong>on</strong>s andparasites).■ The need for a fortificati<strong>on</strong> programme should always be examined in thebroader c<strong>on</strong>text of all the possible opti<strong>on</strong>s for c<strong>on</strong>trolling micr<strong>on</strong>utrient deficiency.It may be that, overall, a combinati<strong>on</strong> of interventi<strong>on</strong>s (i.e. fortificati<strong>on</strong>plus other interventi<strong>on</strong>s) provides the most cost-effective opti<strong>on</strong>. Forinstance, supplementati<strong>on</strong> plus fortificati<strong>on</strong> might be a better way to ensurethat specific populati<strong>on</strong> groups (e.g. pregnant women and young childrenwho are often the most vulnerable groups) are protected against micr<strong>on</strong>utrientdeficiencies than fortificati<strong>on</strong> al<strong>on</strong>e.■ Health authorities looking to initiate a micr<strong>on</strong>utrient fortificati<strong>on</strong> programmeshould not do so <strong>with</strong>out first collecting <strong>food</strong> intake data, supported by ancillaryinformati<strong>on</strong> such as biochemical data <strong>on</strong> nutriti<strong>on</strong>al status. This informati<strong>on</strong>is necessary to justify the programme, to make an informedjudgement about the types and amounts of specific nutrients to include, andto understand which <strong>food</strong>s would make suitable vehicles for fortificati<strong>on</strong>.Given the l<strong>on</strong>g-term effort and investment required to implement and sustainfortificati<strong>on</strong> programmes, and the need to ensure that the outcome is intakesthat are adequate and not excessive, it is essential to make this initial investmentin the collecti<strong>on</strong> of adequate <strong>food</strong> intake data. Trained nutriti<strong>on</strong>ists willbe needed for detailed programme planning, as well as for the subsequentm<strong>on</strong>itoring and evaluati<strong>on</strong> stages, the aim of which is to see how theprogramme has affected the nutrient intakes and nutriti<strong>on</strong>al status of therecipients.In terms of providing reliable informati<strong>on</strong> <strong>on</strong> micr<strong>on</strong>utrient status at the populati<strong>on</strong>level, biochemical and clinical data do, however, have a number of limitati<strong>on</strong>s.Firstly, available resources are usually such that <strong>on</strong>ly a relatively smallnumber of individuals are tested or observed, and those that are sampled arenot always representative of all relevant populati<strong>on</strong> subgroups. Sec<strong>on</strong>dly, some140
7. DEFINING AND SETTING PROGRAMME GOALbiochemical data are difficult to interpret because of c<strong>on</strong>founding factors, suchas the presence of infecti<strong>on</strong>s, or interacti<strong>on</strong>s am<strong>on</strong>g micr<strong>on</strong>utrient deficiencies(see Tables 3.1, 3.4, 3.6, 4.1, 4.3–4.5, 4.7, 4.8, 4.10, 4.11, 4.13–16). Biochemicalindicators of ir<strong>on</strong> status are especially pr<strong>on</strong>e to problems of this nature(Table 3.1). It is especially important to recognize situati<strong>on</strong>s where n<strong>on</strong>-dietaryfactors, such as parasitic infecti<strong>on</strong>s, are likely to be a major cause of observedmicr<strong>on</strong>utrient deficiencies; this will be reflected in a greater severity and prevalenceof deficiencies than would be predicted from dietary data. Under such circumstances,other public health measures – in additi<strong>on</strong> to fortificati<strong>on</strong> – may beneeded to reduce the burden of MNM.A third limitati<strong>on</strong> is a lack of data, either because of the absence of a suitablebiomarker of deficiency or simply because, to date, little investigati<strong>on</strong> has takenplace. This means that the prevalence of many deficiencies suspected of beingrelatively comm<strong>on</strong> (e.g. riboflavin (vitamin B 2 ), vitamin B 12 , zinc and calcium)is not well known (Table 1.2). In some cases, however, evidence of a deficiencyin <strong>on</strong>e micr<strong>on</strong>utrient predicts the existence of deficiencies in others. Forexample, a high prevalence of anaemia and vitamin A deficiency is often accompaniedby zinc, vitamin B 12 and riboflavin (vitamin B 2 ) deficiencies, because theunderlying problem in all cases is an inadequate intake of animal source <strong>food</strong>s(see Chapter 4).7.1.2 Dietary patternsKnowledge of the usual <strong>food</strong>s c<strong>on</strong>sumed can be a useful supplement to biochemicaland clinical evidence of micr<strong>on</strong>utrient deficiencies, and in the absenceof the latter can help pinpoint which micr<strong>on</strong>utrients are most likely to be lackingin the diet. For example, animal source <strong>food</strong>s are the major source of vitaminsA and D, thiamin (vitamin B 1 ), riboflavin (vitamin B 2 ), ir<strong>on</strong>, zinc and calcium,and are the <strong>on</strong>ly source of vitamin B 12 . They also provide an important amountof fat, the presence of which in the diet improves the absorpti<strong>on</strong> of fat-solublevitamins. Populati<strong>on</strong>s <strong>with</strong> a low intake of animal source <strong>food</strong>s are thus likely toexperience deficiencies in some or all of these nutrients.It is comm<strong>on</strong> for the intake of animal source <strong>food</strong>s to be low in disadvantagedpopulati<strong>on</strong>s; sometimes these <strong>food</strong>s are avoided because of religious orother beliefs. Another widespread problem, particularly am<strong>on</strong>g refugees and displacedpopulati<strong>on</strong>s, is inadequate c<strong>on</strong>sumpti<strong>on</strong> of fruits and vegetables and c<strong>on</strong>sequentlylow intakes of vitamin C (ascorbic acid) and folate. In locati<strong>on</strong>s wherephytate or polyphenol intakes are high, the risk of ir<strong>on</strong> and zinc deficienciesincreases because the bioavailability of both of these minerals from <strong>food</strong>s isreduced in the presence of these compounds.At the populati<strong>on</strong> level, <strong>food</strong> balance sheets, such as those produced by theFood and Agriculture Organizati<strong>on</strong> of the United Nati<strong>on</strong>s (FAO), can provide141
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSsome useful informati<strong>on</strong> about usual dietary patterns and also <strong>on</strong> the averagec<strong>on</strong>sumpti<strong>on</strong> of certain <strong>food</strong>s that are rich in micr<strong>on</strong>utrients or in absorpti<strong>on</strong>inhibitors, which in turn can be used to predict probable micr<strong>on</strong>utrient deficiencies.Their main limitati<strong>on</strong> is that, in providing informati<strong>on</strong> <strong>on</strong> the averageintake by the general populati<strong>on</strong>, they do not reflect the distributi<strong>on</strong> of intakesby populati<strong>on</strong> subgroups.7.1.3 Usual dietary intakesAs they are the basis of decisi<strong>on</strong>s about which micr<strong>on</strong>utrients to add to which<strong>food</strong>s and in what amounts, the possessi<strong>on</strong> of quantitative <strong>food</strong> and nutrientintake data is a prerequisite for any <strong>food</strong> fortificati<strong>on</strong> programme. Food intakedata are also needed for predicting the probable impact of potential fortificati<strong>on</strong>interventi<strong>on</strong>s. Food and nutrient intake informati<strong>on</strong> should be available for, orcollected from, different populati<strong>on</strong> groups (e.g. those differing in socioec<strong>on</strong>omicstatus, ethnicity or religious beliefs) and from different physiologicalstatus groups (e.g. children, women).In reality, there is usually a wide range of <strong>food</strong> and nutrient intakes <strong>with</strong>inany given populati<strong>on</strong> subgroup. As is explained in more detail later in thechapter, it is this range or the distributi<strong>on</strong> of usual intakes that is of primaryinterest and which forms the basis for both the planning and the evaluati<strong>on</strong> of<strong>food</strong> fortificati<strong>on</strong> interventi<strong>on</strong>s (see secti<strong>on</strong>s 7.2 and 7.3).7.2 Defining nutriti<strong>on</strong>al goals: basic c<strong>on</strong>ceptsThe main goal of a fortificati<strong>on</strong> programme is to correct inadequate micr<strong>on</strong>utrientintakes through the fortificati<strong>on</strong> of <strong>food</strong>s, thereby preventing, or reducing,the severity and prevalence of micr<strong>on</strong>utrient deficiencies. Interventi<strong>on</strong>s of thisnature can involve either fortifying a single <strong>food</strong> product (e.g. the iodizati<strong>on</strong> ofsalt) or the fortificati<strong>on</strong> of several <strong>food</strong>s.In practice, <strong>food</strong> fortificati<strong>on</strong> programmes are devised so as to achieve a levelof fortificati<strong>on</strong> such that, when the programme is in place, the probability of thenutrient intake being inadequate in a given populati<strong>on</strong> – either insufficient orexcessive – is acceptably low.The dietary goal of fortificati<strong>on</strong> is formally defined in these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> as follows:to provide most (97.5%) of individuals in the populati<strong>on</strong> group(s) at greatest riskof deficiency <strong>with</strong> an adequate intake of specific micr<strong>on</strong>utrients, <strong>with</strong>out causinga risk of excessive intakes in this or other groups.142
7. DEFINING AND SETTING PROGRAMME GOAL7.2.1 The EAR cut-point methodThe approach recommended in these WHO <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for setting fortificantlevels in <strong>food</strong>s is the Estimated Average Requirement (EAR) 1 cut-point method. Thisapproach was proposed some years ago, and is described in detail in a reportby the United States Food and Nutriti<strong>on</strong> Board of the Institute of Medicine(FNB/IOM) <strong>on</strong> dietary reference intakes (333).As its starting point, the EAR cut-point method assumes that the proporti<strong>on</strong>of a populati<strong>on</strong> <strong>with</strong> intakes below the EAR for a given nutrient corresp<strong>on</strong>ds tothe proporti<strong>on</strong> having an inadequate intake of that nutrient (see Figure 7.1).The EAR cut-point approach requires a decisi<strong>on</strong> to be made about the acceptableprevalence of inadequate (and excessive) intakes (often taken to be 2–3%for reas<strong>on</strong>s which are explained more fully below: see secti<strong>on</strong> 7.3.1). Then bycombining informati<strong>on</strong> <strong>on</strong> the range of usual intakes of a populati<strong>on</strong> subgroup<strong>with</strong> informati<strong>on</strong> <strong>on</strong> the nutrient requirements for that subgroup (i.e. the EAR),it is possible to derive a level of <strong>food</strong> fortificati<strong>on</strong> that will give an intake distributi<strong>on</strong>such that usual nutrient intakes meet the requirements of all but a smallspecified proporti<strong>on</strong> of the subgroup. In other words, the method allows its usersto find the additi<strong>on</strong>al intake of micr<strong>on</strong>utrients that would shift the distributi<strong>on</strong>of intakes upwards so that <strong>on</strong>ly a small proporti<strong>on</strong> of the populati<strong>on</strong> group is atrisk of having an inadequate intake. Here the term “subgroup” refers to variousage, gender and physiological status groups of the populati<strong>on</strong> (e.g. pregnant orlactating women). The EAR cut-point methodology is outlined in greater detailin secti<strong>on</strong> 7.3 and is illustrated by means of a worked example.The EAR cut-point method is a simplified, easier to use versi<strong>on</strong> of the probabilitymethod, which requires calculating the probability of inadequacy of intakefor each individual in a populati<strong>on</strong> subgroup, averaging the probabilities, andthen using this average as an estimate of the prevalence of inadequacy (333).These two approaches, the EAR cut-point method and the probability method,give similar results as l<strong>on</strong>g as the assumpti<strong>on</strong>s underlying them are met. For theprobability method, there should be little or no correlati<strong>on</strong> between intake andrequirements, which is assumed to be true for all nutrients but energy. In thecase of the EAR cut-point method, the variati<strong>on</strong> in intake of a nutrient by a populati<strong>on</strong>group should be greater than the variati<strong>on</strong> in the requirement for thisnutrient (also assumed to be true for most nutrients and most groups), and thedistributi<strong>on</strong> of requirements must be symmetrical (believed to be true for allnutrients except ir<strong>on</strong>). Thus for most applicati<strong>on</strong>s and nutrients, either methodis appropriate, <strong>with</strong> the excepti<strong>on</strong> of ir<strong>on</strong> for which <strong>on</strong>ly the probability methodis valid (see secti<strong>on</strong> 7.3.3.1).1The Estimated Average Requirement (EAR) for a micr<strong>on</strong>utrient is defined as the average dailyintake that is estimated to meet the requirements of half of the healthy individuals in a particularlife stage and gender subgroup (332).143
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSFIGURE 7.1An example of a usual intake distributi<strong>on</strong> in which the median intake is at theRNI or RDA (the formerly-used approach)EARRDA = Median of the TargetIntake Distributi<strong>on</strong>Frequency28%Intake distributi<strong>on</strong>54 65Usual intakeSource: adapted from reference (333), <strong>with</strong> the permissi<strong>on</strong> of the United States Nati<strong>on</strong>alAcademy Press.The EAR cut-point approach is different from the past practice of using theRecommended Nutrient Intake (or Recommended Dietary Allowance) of anutrient as the desirable or “target” intake. For reas<strong>on</strong>s that are explained morefully below, the latter approach is valid for deriving the desired nutriti<strong>on</strong>al intakeof an individual, but not that of a populati<strong>on</strong>.7.2.2 Dietary reference values: Estimated Average Requirements,Recommended Nutrient Intakes and upper limits7.2.2.1 Recommended Nutrient IntakesDietary requirements for specific micr<strong>on</strong>utrients, aimed at minimizing the riskof nutrient deficit or excess, have been specified by various nati<strong>on</strong>al and internati<strong>on</strong>albodies, including FAO and WHO. The Recommended Nutrient Intake(RNI) is defined by FAO/WHO as the daily dietary intake level that is sufficientto meet the nutrient requirement of almost all (i.e. 97–98%) healthy individualsin a particular age, gender and physiological status group (93). For most nutrients,the RNI is set at about 2 standard deviati<strong>on</strong>s higher than the averageamount required by a populati<strong>on</strong> group (i.e. the EAR), in order that the requirementsof almost every pers<strong>on</strong> in the group are met. The standard deviati<strong>on</strong> (orcoefficient of variati<strong>on</strong>) 1 of the requirement for each nutrient varies <strong>with</strong> age,gender and physiological status but for most nutrients and subgroups is between10% and 20%.Table 7.1 lists published FAO/WHO RNI values for all micr<strong>on</strong>utrientscovered by these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for selected age and gender groups (93). The1The coefficient of variati<strong>on</strong> is the standard deviati<strong>on</strong> divided by the mean, expressed as a percentage.144
7. DEFINING AND SETTING PROGRAMME GOALTABLE 7.1FAO/WHO Recommended Nutrient Intakes (RNIs) for selected populati<strong>on</strong> subgroupsNutrient (unit) 1–3 years 4–6 years 19–50 years, female Pregnant women, Lactating women, 19–50 years, malesec<strong>on</strong>d trimester 0–3 m<strong>on</strong>thsVitamin A (µg RE) a 400 450 500 800 850 600Vitamin D (µg) b 5 5 5 5 5 5Vitamin E (mg α-tocopherol) 5.0 5.0 7.5 7.5 7.5 10.0Vitamin C (mg) 30 30 45 55 70 45Thiamine (vitamin B1) (mg) 0.5 0.6 1.1 1.4 1.5 1.2Riboflavin (vitamin B2) (mg) 0.5 0.6 1.1 1.4 1.6 1.3Niacin (vitamin B3) (mg NE) 6 8 14 18 17 16Vitamin B6 (mg) 0.5 0.6 1.3 1.9 2.0 1.3Folate (µg DFE) c 150 200 400 600 500 400Vitamin B12 (µg) 0.9 1.2 2.4 2.6 2.8 2.4Ir<strong>on</strong> (mg) d■ 15% bioavailability 3.9 4.2 19.6 >50.0 10.0 9.1■ 10% bioavailability 5.8 6.3 29.4 >50.0 15.0 13.7■ 5% bioavailability 11.6 12.6 58.8 >50.0 30.0 27.4Zinc (mg) e■ High bioavailability 2.4 2.9 3.0 4.2 5.8 4.2■ Moderate bioavailability 4.1 4.8 4.9 7.0 9.5 7.0■ Low bioavailability 8.3 9.6 9.8 14.0 19.0 14.0Calcium (mg) 500 600 1 000 1 000 1 000 1 000Selenium (µg) 17 22 26 28 35 34Iodine (µg) 90 90 150 200 200 150a1 RE = 1 µg retinol = 12 µg β-carotene or 24 µg other provitamin A carotenoids. In oil, the c<strong>on</strong>versi<strong>on</strong> factor for vitamin A (retinol): β-carotene is 1 : 2.The corresp<strong>on</strong>ding c<strong>on</strong>versi<strong>on</strong> factor for synthetic β-carotene is uncertain, but a factor of 1 : 6 is generally c<strong>on</strong>sidered to be reas<strong>on</strong>able. 1 µg RE =3.33 IU vitamin A.bIn the absence of adequate exposure to sunlight, as calciferol (1 µg calciferol = 40 IU vitamin D).c1 DFE = Dietary folate equivalent = 1 µg <strong>food</strong> folate = 0.6 µg folic acid from fortified <strong>food</strong>s, which means that 1 µg folic acid = 1.7 DFE.dThe RNI depends <strong>on</strong> the compositi<strong>on</strong> of the diet. For a diet rich in vitamin C and animal protein, the bioavailability of ir<strong>on</strong> is 15%; for diets rich incereals but including sources of vitamin C, bioavailability is 10%, and for diets low in vitamin C and animal protein, bioavailability is reduced to 5%.eThe RNI depends <strong>on</strong> the compositi<strong>on</strong> of the diet. The bioavailability of zinc is high from diets rich in animal protein, moderate from diets rich inlegumes and pulses or diets that include fermented cereals, and low from diets poor in animal protein or zinc-rich plant <strong>food</strong>s.Source: reference (93), which also provides values for other age and gender groups. T.145
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSBOX 7.2FAO/WHO RNIs: comparis<strong>on</strong>s <strong>with</strong> dietary re f e rence valuesd e fined by other bodies1. Food and Nutriti<strong>on</strong> Board, Institute of Medicine (FNB/IOM), United States ofAmericaThe FAO/WHO RNI is c<strong>on</strong>ceptually equivalent to the Recommended DietaryAllowance (RDA), <strong>on</strong>e of the four levels of dietary reference intakes used inCanada and the United States of America. The other three values are the EstimatedAverage Requirement (EAR), the Adequate Intake (AI), and the TolerableUpper Level (UL) 1 .2. Department of Health, United KingdomThe FAO/WHO RNI is c<strong>on</strong>ceptually similar to the Reference Nutrient Intake(RNI), <strong>on</strong>e of the four dietary reference values used in the United Kingdom(334). The others are the Estimated Average Requirement, the Lower ReferenceNutrient Intake (a c<strong>on</strong>cept that is unique to the United Kingdom) and the SafeIntake, which is c<strong>on</strong>ceptually similar to the Adequate Intake as defined by theUnited States FNB/IOM.3. Scientific Committee for Foods, Commissi<strong>on</strong> of the European CommunityThe European Community currently uses three reference values: the Populati<strong>on</strong>Requirement Intake (PRI), which is c<strong>on</strong>ceptually equivalent to FAO/WHO RNI,the Average Requirement (AR) and the Lower Threshold Intake (LTI) (335).FAO/WHO RNI values are broadly similar to dietary reference values definedby other nati<strong>on</strong>al and internati<strong>on</strong>al bodies. Various dietary reference values incomm<strong>on</strong> usage, and their equivalence, are summarized in Box 7.2.For the majority of micr<strong>on</strong>utrients, the highest recommended intakes are foradult males, the notable excepti<strong>on</strong> being ir<strong>on</strong>. Nevertheless, this populati<strong>on</strong> subgroupusually has the lowest risk of micr<strong>on</strong>utrient deficiencies due to its higher<strong>food</strong> intake and its lower micr<strong>on</strong>utrient requirements per unit body weight. Individualsat most risk of not meeting their RNIs are infants, young children andwomen of reproductive age, especially pregnant and lactating women. Some ofthese groups (e.g. pregnant or lactating women) may even have higher requirementsfor specific nutrients than do adult men.7.2.2.2 Calculating Estimated Average Requirements from RecommendedNutrient IntakesAlthough they form the basis of most RNIs (which are usually set at 2 standarddeviati<strong>on</strong>s above the corresp<strong>on</strong>ding EAR for any given populati<strong>on</strong> subgroup),1For further informati<strong>on</strong> relating to the work and publicati<strong>on</strong>s of the Food and Nutriti<strong>on</strong> Board,please refer to the web site of the Nati<strong>on</strong>al Academies Press (http://www.nap.edu).146
7. DEFINING AND SETTING PROGRAMME GOALFAO/WHO do not routinely publish EAR values. However, the FAO/WHORNIs, or equivalent recommendati<strong>on</strong>s made by other countries or regi<strong>on</strong>s, canbe easily c<strong>on</strong>verted into EARs by the applicati<strong>on</strong> of appropriate c<strong>on</strong>versi<strong>on</strong>factors. The c<strong>on</strong>versi<strong>on</strong> factors, which are presented for the micr<strong>on</strong>utrientscovered by these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> in Annex C, are the equivalent of subtracting 2standard deviati<strong>on</strong>s from the RNI. For example, the standard deviati<strong>on</strong> of therequirement for vitamin A by 1-3-year-old children is 20%; dividing the relevantRNI (400µg RE) by 1.4 (i.e. 1 + (2 × 0.2)) gives an EAR of 286 µg RE. TheEARs corresp<strong>on</strong>ding to RNIs given in Table 7.1, and calculated in this way, arelisted in Table 7.2.7.2.2.3 Upper levels of intakeThe most appropriate reference value for determining whether or not themicr<strong>on</strong>utrient intakes of populati<strong>on</strong> subgroups are safe, i.e. do not reach levelsat which there is any risk of excessive intake, is the Tolerable Upper Intake Level(UL). The UL is the highest average intake that will not pose a risk of adversehealth effects for virtually any<strong>on</strong>e in the populati<strong>on</strong>. The risk of adverse effectsincreases at intakes above the UL. The risks of excessive intakes are describedin detail by FAO/WHO (93), and the United States FNB/IOM (332,333).Like EARs and RNIs, ULs vary by age and gender but tend to be lower foryoung children and pregnant women. ULs for a range of micr<strong>on</strong>utrients aregiven in Table 7.3. For those micr<strong>on</strong>utrients for which FAO/WHO have not recommendeda UL (i.e. ir<strong>on</strong>, folate, fluoride and iodine), the values given in thetable are based <strong>on</strong> recommendati<strong>on</strong>s of either the United States FNB/IOM orthe Scientific Committee for Food of the European Community.7.3 Using the EAR cut-point method to set goals and toevaluate the impact and safety of fortificati<strong>on</strong>In reality, there is usually a wide range of intakes of a nutrient <strong>with</strong>in a populati<strong>on</strong>subgroup. This range of usual intakes must be measured and used as thebasis for planning and evaluati<strong>on</strong>. As previously menti<strong>on</strong>ed, the goal of fortificati<strong>on</strong>is to shift the distributi<strong>on</strong> of usual nutrient intakes of a target populati<strong>on</strong>upwards so that <strong>on</strong>ly a small proporti<strong>on</strong> of the populati<strong>on</strong> is at risk of havingan inadequate intake, but not so far that those who c<strong>on</strong>sume larger amounts ofthe <strong>food</strong> vehicle will be at risk of an excessive intake. The median of the newusual intake distributi<strong>on</strong> is referred to as the “target median intake”. It thusfollows that <strong>on</strong>e of the first decisi<strong>on</strong>s that will need to be made when planningfortificati<strong>on</strong> interventi<strong>on</strong>s is what is an acceptable prevalence of inadequacy,both for low as well as for high intakes.147
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 7.2Estimated Average Requirements (calculated values) based <strong>on</strong> FAO/WHO Recommended Nutrient IntakesNutrient (unit) 1–3 years 4–6 years 19–50 years, female Pregnant women, Lactating women, 19–50 years, malesec<strong>on</strong>d trimester 0–3 m<strong>on</strong>thsVitamin A (µg RE) a 286 321 357 571 607 429Vitamin D (µg) b 5 5 5 5 5 5Vitamin E (mg α-tocopherol) 4 4 6 6 6 8Vitamin C (mg) 25 25 37 46 58 37Thiamine (vitamin B1) (mg) 0.4 0.5 0.9 1.2 1.3 1.0Riboflavin (vitamin B2) (mg) 0.4 0.5 0.9 1.2 1.3 1.1Niacin (vitamin B3) (mg NE) 5 6 11 14 13 12Vitamin B6 (mg) 0.4 0.5 1.1 1.6 1.7 1.1Folate (µg DFE) c 120 160 320 480 400 320Vitamin B12 (µg) 0.7 1.0 2.0 2.2 2.3 2.0Ir<strong>on</strong> (mg) d■ 15% bioavailability 3.9 e 4.2 e 19.6 e >40.0 7.8 7.2■ 10% bioavailability 5.8 e 6.3 e 29.4 e >40.0 11.7 10.8■ 5% bioavailability 11.6 e 12.6 e 58.8 e >40.0 23.4 21.6Zinc (mg) f■ High bioavailability 2.0 2.4 2.5 3.5 4.8 3.5■ Moderate bioavailability 3.4 4.0 4.1 5.8 7.9 5.8■ Low bioavailability 6.9 8.0 8.2 11.7 15.8 11.7Calcium (mg) 417 500 833 833 833 833Selenium (µg) 14 17 22 23 29 28Iodine (µg) 64 64 107 143 143 107a1 RE = 1 µg retinol = 12 µg β-carotene or 24 µg other provitamin A carotenoids. In oil, the c<strong>on</strong>versi<strong>on</strong> factor for vitamin A (retinol): β-carotene is 1 : 2.The corresp<strong>on</strong>ding c<strong>on</strong>versi<strong>on</strong> factor for synthetic β-carotene is uncertain, but a factor of 1 : 6 is generally c<strong>on</strong>sidered to be reas<strong>on</strong>able. 1 µg RE =3.33 IU vitamin A.bIn the absence of adequate exposure to sunlight, as calciferol. 1 µg calciferol = 40 IU vitamin D.c1 DFE = Dietary folate equivalent = 1 µg <strong>food</strong> folate = 0.6 µg folic acid from fortified <strong>food</strong>s, which means that 1 µg folic acid = 1.7 DFE.dThe RNI and thus the calculated EAR depends <strong>on</strong> the compositi<strong>on</strong> of the diet. For a diet rich in vitamin C and animal protein, the bioavailability ofir<strong>on</strong> is 15%; for diets rich in cereals but including sources of vitamin C, bioavailability is 10%, and for diets low in vitamin C and animal protein,bioavailability is reduced to 5%.eEARs cannot be calculated from RNIs for these age groups because of the skewed distributi<strong>on</strong> of requirements for ir<strong>on</strong> by young children and menstruatingwomen. Instead, the corresp<strong>on</strong>ding RNI values are given.fThe RNI and thus the calculated EAR depends <strong>on</strong> the compositi<strong>on</strong> of the diet. The bioavailability of zinc is high from diets rich in animal protein,moderate from diets rich in legumes and pulses or diets that include fermented cereals, and low from diets poor in animal protein or zinc-rich plant<strong>food</strong>s.Source: calculated from FAO/WHO RNIs, using the factors given in Annex C of these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>.148
7. DEFINING AND SETTING PROGRAMME GOALTABLE 7.3Tolerable Upper Intake Levels (ULs)Nutrient (unit) a 1–3 years 4–8 years 9–13 years 19–70 yearsVitamin A (µg RE) b 600 900 1 700 3 000Vitamin D (µg) c 50 50 50 50Vitamin E (mg α-tocopherol) 200 300 600 1 000Vitamin C (mg) 400 650 1 200 1 000 dNiacin (vitamin B 3 )(mg NE) e 10 15 20 35Vitamin B 6 (mg) 30 40 60 100Folic acid (µg DFE) f 300 400 600 1 000Choline (mg) 1 000 1 000 2 000 3 500Ir<strong>on</strong> (mg) 40 40 40 45Zinc (mg) 7 12 23 45 gCopper (mg) 1 3 5 10Calcium (mg) 2 500 2 500 2 500 3 000 hPhosphorus (mg) 3 000 3 000 4 000 4 000Manganese (mg) 2 3 6 11Molybdenum (µg) 300 600 1 100 2 000Selenium (µg) 90 150 280 400Iodine (µg) 200 300 600 1 100Fluoride (µg) 1 300 2 200 10 000 10 000aAlthough no UL is specified for arsenic, silic<strong>on</strong> and vanadium, there is no justificati<strong>on</strong> foradding these substances to <strong>food</strong>s.bRefers to preformed vitamin A <strong>on</strong>ly (i.e. esters of retinol). 1 µg RE = 3.33 IU vitamin A.cAs calciferol, where 1 µg calciferol = 40 IU vitamin D.dThe United States Food and Nutriti<strong>on</strong> Board of the Institute of Medicine recommends a ULof 2 000 mg vitamin C/day for adults.eBased <strong>on</strong> the flushing effects of nicotinic acid. If niacinamide is used as the fortificant, theUL would be much higher. A UL for adults of 900 mg niacinamide/day has been recommendedby the European Commissi<strong>on</strong> (319).fRefers to folic acid derived from fortified <strong>food</strong>s, or supplemental folic acid.gThe United States Food and Nutriti<strong>on</strong> Board of the Institute of Medicine recommends a ULof 40 mg zinc/day for adults (91).hThe United States Food and Nutriti<strong>on</strong> Board of the Institute of Medicine recommends a ULof 2 500 mg calcium/day for adults (193).Sources: adapted from references (91,93). FAO/WHO have <strong>on</strong>ly recommended ULs for vitaminsA, B 3 (niacin), B 6 , C, D and E, calcium, selenium and zinc for adults. The remaining values arethose recommended by the United States Food and Nutriti<strong>on</strong> Board of the Institute of Medicine.7.3.1 Deciding <strong>on</strong> an acceptable prevalence of low intakesThree different ways of planning an intake distributi<strong>on</strong> for a hypothetical nutrient,which has an EAR of 54 and an RDA of 65, are compared below (Figures7.1–7.3). For simplicity, the intake distributi<strong>on</strong>s shown in these examples arenormally distributed, although in reality, intake distributi<strong>on</strong>s are usually slightlyskewed.149
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSFIGURE 7.2An example of a usual intake distributi<strong>on</strong> in which <strong>on</strong>ly 2.5% of the grouphave intakes below the RNI (or RDA)EARRDAMedian of the TargetIntake Distributi<strong>on</strong>Frequency2.5%Intake distributi<strong>on</strong>54 65Usual intakeSource: adapted from reference (333), <strong>with</strong> the permissi<strong>on</strong> of the United States Nati<strong>on</strong>alAcademy Press.101FIGURE 7.3An example of a usual intake distributi<strong>on</strong> in which 2.5% of the group haveintakes below the EAR (the recommended approach)EARRDAMedian of the TargetIntake Distributi<strong>on</strong>Frequency2.5%Intake distributi<strong>on</strong>54 65Usual intake90Source: adapted from reference (333), <strong>with</strong> the permissi<strong>on</strong> of the United States Nati<strong>on</strong>alAcademy Press.Scenario 1Until relatively recently, nutriti<strong>on</strong>ists used the RNI as the basis for dietary planningand evaluati<strong>on</strong>, and had as their optimum goal a populati<strong>on</strong> intake distributi<strong>on</strong>in which the mean or median nutrient intakes for populati<strong>on</strong> subgroupsmet the relevant RNI (or its North American equivalent, the RDA). Assumingthat the nutrient intakes are normally distributed, it is clear from Figure 7.1that according to this scenario, half of the populati<strong>on</strong> subgroup would haveintakes that were below the RNI or RDA, while the intakes of the other halfwould be higher than the RNI (or RDA). More importantly, a relatively largepercentage of the populati<strong>on</strong> subgroup would have usual intakes below the EAR(28% in this example). A target median intake set at the RNI is now generally150
7. DEFINING AND SETTING PROGRAMME GOALc<strong>on</strong>sidered to result in an unacceptably high prevalence of inadequate lowintakes.Scenario 2An approach that plans for the usual intakes of all but 2.5% of the group to beabove the RNI (or RDA) is equally unacceptable. For this to happen, the targetmedian intake of the group would need to be set at a very high level, that is tosay, at almost twice the RNI (or RDA). Virtually no intakes would be below theEAR (Figure 7.2). Adopting this approach increases the risk of exceeding theUL (if there is <strong>on</strong>e), and may also result in adverse effects <strong>on</strong> the organlolepticproperties of <strong>food</strong>s (due to the relatively high levels of added fortificants). Onbalance, such an approach is widely regarded as being unrealistic, costly, inefficientand potentially risky.Scenario 3The recommended strategy is thus to shift the usual distributi<strong>on</strong> of intakesupwards so that the intake of each nutrient is at least at the EAR for all except2–3% of the target populati<strong>on</strong> group (Figure 7.3). In this example, if themicr<strong>on</strong>utrient interventi<strong>on</strong>s are planned so that <strong>on</strong>ly 2–3% of the group have anintake less than the EAR, the target median intake would have to be about 1.5times the RNI (or RDA) and approximately 20% of the populati<strong>on</strong> group wouldhave intakes below the RNI (or RDA). In other words, the program would besatisfactory when most individuals of the populati<strong>on</strong> (97–98%) satisfy the EAR,which may be similar to saying that most individuals of the populati<strong>on</strong> satisfy80% of RNI.7.3.2 Calculating the magnitude of micr<strong>on</strong>utrient additi<strong>on</strong>sThis is the secti<strong>on</strong> of the <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> in which the applicati<strong>on</strong> of the EAR cutpointmethod to the calculati<strong>on</strong> of fortificati<strong>on</strong> levels (i.e. the amount of fortificantrequired to bring about the desired upwards shift in the distributi<strong>on</strong> ofusual intakes) is explained in greater detail.There are four steps: the first involvesexamining the prevalence of inadequate intakes of each nutrient in specific populati<strong>on</strong>groups. Having identified which populati<strong>on</strong> subgroups have the highestprevalence of inadequate intakes (step 2) and estimated the usual c<strong>on</strong>sumpti<strong>on</strong>of the chosen <strong>food</strong> vehicle by this group (step 3), the final step is to calculatethe reducti<strong>on</strong> in the prevalence of inadequate intakes (i.e. the proporti<strong>on</strong> belowthe EAR) and the risk of excessive intakes (i.e. the proporti<strong>on</strong> above the UL)that would be expected to occur at different levels of fortificati<strong>on</strong>. The methodologyis illustrated <strong>with</strong> reference to a hypothetical case in which wheat flour isto be fortified <strong>with</strong> vitamin A.151
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSStep 1. Observe the usual distributi<strong>on</strong> of nutrient intakes in specificpopulati<strong>on</strong> subgroupsAs menti<strong>on</strong>ed in secti<strong>on</strong> 7.1.3, programme planning requires quantitativedietary intake data, initially for evaluating the current level of nutrient intakes ina populati<strong>on</strong> subgroup. This informati<strong>on</strong> is also necessary for estimating theamount of micr<strong>on</strong>utrient that would need to be supplied by a micr<strong>on</strong>utrientdeliveryprogramme, and for predicting the impact of adding different amountsof micr<strong>on</strong>utrients to different <strong>food</strong>s.It is not necessary to c<strong>on</strong>duct large surveys, although intake data should becollected from a stratified, representative sample of the whole target area. Ideally,all populati<strong>on</strong> subgroups should be represented. Collecting informati<strong>on</strong> fromabout 200 individuals in each populati<strong>on</strong> subgroup <strong>with</strong> the highest risk of deficiency(e.g. preschool-aged children and reproductive-age women in the lowestrural income group) and the highest risk of excess (e.g. men in the highest urbanincome group in the case of staple <strong>food</strong>s), and any other groups that are locallyrelevant, should provide sufficient valid informati<strong>on</strong>. It is helpful to select populati<strong>on</strong>subgroups <strong>with</strong> similar age ranges as those for which EARs are defined(see Table 7.2). Pregnant and lactating women should usually be treated as separatepopulati<strong>on</strong> subgroups.Quantitative <strong>food</strong> intake data is usually obtained by 24-hour recall surveytechniques or a combinati<strong>on</strong> of weighed/measured <strong>food</strong> intakes and recall,depending <strong>on</strong> what is locally possible. The distributi<strong>on</strong> of usual intakes obtainedin this way will be unrealistically wide, reflecting the fact that individuals mayeat atypically small or large amounts <strong>on</strong> the day their intake was measured. Bycollecting at least 2 (preferably n<strong>on</strong>-c<strong>on</strong>secutive) days worth of <strong>food</strong> intake datafor each individual, it is possible to estimate the day-to-day or intra-individualvariability in intake, and adjust the distributi<strong>on</strong> accordingly. For more detailedinformati<strong>on</strong> about the statistical techniques that are available for reducing dayto-dayvariability in dietary intake data, readers are referred to relevant texts <strong>on</strong>this subject (332,333,336,337) 1 . If two days of dietary data per pers<strong>on</strong> are notavailable, or cannot be collected for what is felt to be a representative sample ofthe target populati<strong>on</strong> groups, it will then be necessary to use an estimate of dayto-dayvariability obtained elsewhere, but preferably <strong>on</strong>e that has been obtainedfrom a similar populati<strong>on</strong>. A study of the variability in nutrient intakes in a populati<strong>on</strong>in Malawi has recently been published and may be useful in this regard(338).Allowing for day-to-day variati<strong>on</strong> usually has the effect of narrowing theintake distributi<strong>on</strong>, so that fewer individuals will be below the EAR (and above1The statistical method for reducing variability in intake data described by the Food and Nutriti<strong>on</strong>Board of the Institute of Medicine (332) is also readable at the following web site:http://www.nap.edu/catalog/9956.html).152
7. DEFINING AND SETTING PROGRAMME GOALthe UL). If some form of adjustment for variability is not made, the prevalenceof inadequacies could be incorrectly estimated because the distributi<strong>on</strong> does notreflect the usual intakes of the individuals in the group.Having collated informati<strong>on</strong> <strong>on</strong> the quantities of various <strong>food</strong>s c<strong>on</strong>sumed,local, regi<strong>on</strong>al or internati<strong>on</strong>al <strong>food</strong> compositi<strong>on</strong> tables can then be used toc<strong>on</strong>vert such data into amounts of nutrients c<strong>on</strong>sumed. If local informati<strong>on</strong> isnot adequate, there are several internati<strong>on</strong>al and regi<strong>on</strong>al databases that havebeen established expressly for this purpose. The United Nati<strong>on</strong>s group,INFOODS 1 , is a repository of databases of this type and also serves as a resourcefor organizati<strong>on</strong>s and individuals interested in <strong>food</strong> compositi<strong>on</strong> data. TheINFOODS web site provides links to various software programmes, forexample, the WorldFood Dietary Assessment System 2 , which can be used to calculatenutrient intakes from <strong>on</strong>e-day dietary informati<strong>on</strong>. The results, i.e. thedistributi<strong>on</strong> of usual intake, for each nutrient and for each populati<strong>on</strong> group, aredisplayed as percentiles.E x a m p l eAnalysis of quantitative <strong>food</strong> intake survey data, according to the methodologyoutlined above, reveals that in adult women the distributi<strong>on</strong> of vitamin A intakesis such that the median c<strong>on</strong>sumpti<strong>on</strong> is 240 µg RE per day. 5% of adult womenhave intakes that are less than 120 µg RE per day and 25% have vitamin Aintakes that are below 200 µg RE per day. See Table 7.4.Step 2. Identify the populati<strong>on</strong> subgroups at greatest risk of inadequateintakes of specific micr<strong>on</strong>utrientsCertain subgroups of the populati<strong>on</strong> (usually children and women) tend to beat higher risk of having an inadequate intake of specific nutrients. On completi<strong>on</strong>of step 1, it will be evident which subgroups have the highest prevalence ofinadequate intakes for which nutrients. It is important to identify which populati<strong>on</strong>groups are at greatest risk so that a micr<strong>on</strong>utrient-delivery programmecan target these groups.12INFOODS is the acr<strong>on</strong>ym of the Internati<strong>on</strong>al Network of Food Data Systems, which was establishedin 1984, <strong>on</strong> the recommendati<strong>on</strong> of an internati<strong>on</strong>al group c<strong>on</strong>vened under the auspicesof the United Nati<strong>on</strong>s University. Its goal is to stimulate and coordinate efforts to improve thequality and availability of <strong>food</strong> analysis data worldwide and to make sure that accurate and reliable<strong>food</strong> compositi<strong>on</strong> data are readily accessible by all. Further informati<strong>on</strong> can be obtained fromthe web site: http://www.fao.org/in<strong>food</strong>s (accessed 15 March 2005).Software links can be accessed via the web site: http://www.fao.org/in<strong>food</strong>s/software_en.stm,(accessed 15 March 2005).153
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 7.4Predicting the effect <strong>on</strong> the intake distributi<strong>on</strong>s of adult women of fortifying wheat flour <strong>with</strong> different levels of vitamin A aPercentile Distributi<strong>on</strong> of Distributi<strong>on</strong> of vitamin A intake from all sources Vitamin A intake in relati<strong>on</strong> to the EARusual wheat (µg/day) and the ULc<strong>on</strong>sumpti<strong>on</strong>(g/day)Before After After Before After Afterfortificati<strong>on</strong> fortificati<strong>on</strong> at fortificati<strong>on</strong> at fortificati<strong>on</strong> fortificati<strong>on</strong> fortificati<strong>on</strong>a level of a level of at a level of at a level of3 mg/kg 5 mg/kg 3 mg/kg 5 mg/kgGroup at risk of deficiency5 30 120 210 270 – – –10 45 160 295 385 – – *25 120 200 560 800 – * *50 180 240 780 1 140 – * *Group at risk of excess75 240 600 1 320 1 800 * * *90 300 1 000 1 900 2 500 * * *95 360 1 250 2 330 3 050 * * +Resulting prevalence of inadequacy and risk of toxicityProporti<strong>on</strong> of women <strong>with</strong> intakes below the EAR (%) 65 15 8Proporti<strong>on</strong> of women <strong>with</strong> intakes above the UL (%) 0 2 6–, intake is below the EAR; *, intake is above the EAR but below the UL; +, intake above the UL.aFor vitamin A an EAR of 357 µg/day is assumed. This is derived from the FAO/WHO RNI for this vitamin of 500 µg/day, to which a c<strong>on</strong>versi<strong>on</strong> factorof 1.4 has been applied (see Annex C). The UL is 3 000 µg/day. If β-carotene were used rather than preformed vitamin A (retinol), there would be noUL.154
7. DEFINING AND SETTING PROGRAMME GOALE x a m p l eIn this example, 65% of adult women were found to have inadequate intakes ofvitamin A, i.e. intakes that were below the EAR for this vitamin (357 µg/day). Theproporti<strong>on</strong> of women who were at risk of exceeding the UL (3 000 µg/d) wasvery small (Table 7.4).Step 3. Measure the usual amount of the intended <strong>food</strong> vehicle(s) thatis c<strong>on</strong>sumed by the populati<strong>on</strong> subgroup at greatest risk ofinadequate or excessive intakesIt is important that <strong>food</strong> vehicle c<strong>on</strong>sumpti<strong>on</strong> estimates are obtained not just forthose subgroups that have been found to have the highest prevalence of inadequateintakes in step 2, but also for those subgroups <strong>with</strong> the highest levels ofc<strong>on</strong>sumpti<strong>on</strong> (i.e. those at greatest risk of excessive intakes). This informati<strong>on</strong>will be used to predict the effects of different levels of fortificati<strong>on</strong> <strong>on</strong> the totalintake of the nutrient (see step 4).E x a m p l eHypothetical values for the usual c<strong>on</strong>sumpti<strong>on</strong> of wheat by adult women, acrossthe percentiles of vitamin A intake, are included in Table 7.4. The median c<strong>on</strong>sumpti<strong>on</strong>of wheat is 180 g/day. Ideally, the intake of the proposed <strong>food</strong> vehicleneeds to be higher in the populati<strong>on</strong> groups <strong>with</strong> the highest prevalence of inadequateintakes, and lower in the relatively well-nourished groups. This wouldminimize the risk of the vitamin A intakes of the high wheat c<strong>on</strong>sumers becomingexcessive. Unfortunately, in countries in which wheat flour tends to be c<strong>on</strong>sumedin larger amounts by wealthier (i.e. better-nourished) individuals, this isunlikely to be the case. Although Table 7.4 presents the case for adult women,it is important to point out here that the adult males are usually the group thathave the highest c<strong>on</strong>sumpti<strong>on</strong> of staple <strong>food</strong>s.Step 4. Simulate the effect of adding different levels of nutrient(s) to the<strong>food</strong> vehicleSimulating the effect of micr<strong>on</strong>utrient additi<strong>on</strong>s (by recalculating the distributi<strong>on</strong>of vitamin A intakes but this time assuming that the wheat flour c<strong>on</strong>tainsan additi<strong>on</strong>al 3 or 5 mg/kg vitamin A) helps to identify the most appropriate levelof fortificati<strong>on</strong> for a given <strong>food</strong> vehicle, i.e. a level that prevents deficiency in apopulati<strong>on</strong> at risk, yet avoids a high proporti<strong>on</strong> of very high intakes.155
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSE x a m p l eThe data in Table 7.4 show the effect of fortifying all wheat flour <strong>with</strong> vitamin A(as retinol), at a level of 3 and 5 mg/kg, <strong>on</strong> the distributi<strong>on</strong> of total vitamin Aintakes in adult women, the populati<strong>on</strong> subgroup identified as being at high riskof vitamin A deficiency. Prior to fortificati<strong>on</strong>, the prevalence of inadequateintakes in this group was 65% (see step 2).At a fortificati<strong>on</strong> level of 3 mg/kg of wheat, the prevalence of inadequate intakes(that is, the proporti<strong>on</strong> of the group <strong>with</strong> intakes below the EAR of 357 µg/day)would fall from the pre-fortificati<strong>on</strong> level of about 65% (50–75%) to 15%(10–25%). In other words, as a result of fortificati<strong>on</strong>, about 50% of adult womenhave moved from having an inadequate to an adequate vitamin A intake. If thefortificati<strong>on</strong> level is increased to 5 mg/kg of wheat, <strong>on</strong>ly 8% of women wouldhave an inadequate intake. However, at the 5 mg/kg fortificati<strong>on</strong> level, thehighest 6% of wheat c<strong>on</strong>sumers would have a total vitamin A intake that mightexceed the UL of 3000 µg/day.Given that calculati<strong>on</strong> does not c<strong>on</strong>sider vitamin A intake by adult males,, it maybe better to select the 3 mg/kg fortificati<strong>on</strong> level and then find another <strong>food</strong>vehicle to fortify or another delivery mechanism to meet the shortfall in vitaminA intake in the 15% of women whose intake remains unsatisfied through thec<strong>on</strong>sumpti<strong>on</strong> of fortified wheat flour. Decisi<strong>on</strong>s of this nature can <strong>on</strong>ly be made<strong>on</strong> the basis of local informati<strong>on</strong>, and <strong>with</strong> due c<strong>on</strong>siderati<strong>on</strong> of the potentialrisks associated <strong>with</strong> excessive intakes of vitamin A (see secti<strong>on</strong> 7.5).Step 4 would need to be repeated in order to determine the appropriate fortificati<strong>on</strong>level for wheat flour <strong>with</strong> vitamin A for any other at-risk populati<strong>on</strong>groups identified in step 2. Steps 3 and 4 would then be repeated for any othernutrients being c<strong>on</strong>sidered for the fortificati<strong>on</strong> programme, in which case thecorresp<strong>on</strong>ding EARs and current nutrient intake distributi<strong>on</strong> data would beneeded.7.3.3 Adaptati<strong>on</strong>s to the EAR cut-point methodology for specific nutrients7.3.3.1 Ir<strong>on</strong>The EAR cut-point approach cannot be used to estimate the prevalence of inadequateir<strong>on</strong> intakes in some populati<strong>on</strong> subgroups, notably children, menstruatingadolescents and adult women, <strong>on</strong> account of the fact that their requirementsfor ir<strong>on</strong> are not normally distributed (see secti<strong>on</strong> 7.2.1). The requirements ofmenstruating adolescents and adult women are not normally distributed largelybecause of the skewed distributi<strong>on</strong> of their ir<strong>on</strong> losses, and of menstrual lossesin particular (91). Assuming for present purposes that a coefficient of variati<strong>on</strong>(CV) greater than 40% indicates a skewed distributi<strong>on</strong>, then the populati<strong>on</strong>156
7. DEFINING AND SETTING PROGRAMME GOALgroups <strong>with</strong> skewed requirements for ir<strong>on</strong> are as follows (based <strong>on</strong> data fromthe United States FNB/IOM (91):— children, aged 1–3 years (CV = 67%);— children, aged 4–8 years (CV = 75%);— menstruating adolescents, aged 14–18 years (CV = 45%);— menstruating women (CV = 63%).For the other populati<strong>on</strong> groups, the CV of the distributi<strong>on</strong> of ir<strong>on</strong> requirementsis 30% or less.For those groups <strong>with</strong> skewed requirements, an alternative approach to theEAR cut-point method must be used, namely a full probability approach. Table7.5 gives the probability of an ir<strong>on</strong> intake being inadequate at a given range ofusual ir<strong>on</strong> intake for the populati<strong>on</strong> subgroups of interest, i.e. young childrenand menstruating females. Using these values, it is possible to calculate theprevalence of inadequate intakes in a populati<strong>on</strong> subgroup from estimates ofthe percentage of the group <strong>with</strong> intakes in a given intake range (note that thebioavailability of ir<strong>on</strong> from the usual diet must also be known). For each intakerange, a prevalence of inadequacy is obtained by multiplying the percentage ofthe group <strong>with</strong> intakes in that range by the probability of inadequacy. Summingthe prevalences of inadequacy in each intake range provides an estimate of thetotal prevalence of inadequacy for the populati<strong>on</strong> group of interest.To illustrate the applicati<strong>on</strong> of the probability method (using the data in Table7.5), the prevalence of inadequate ir<strong>on</strong> intakes in a populati<strong>on</strong> of adult menstruatingwomen, c<strong>on</strong>suming a diet from which the ir<strong>on</strong> bioavailability is 5%, iscalculated in Table 7.6. For instance, according to Table 7.5, the women in thelowest ir<strong>on</strong> intake range (i.e. less than 15 mg per day) have a probability of inadequacyof 1.0, which means that all the women in this group have ir<strong>on</strong> intakesthat are less than their requirements. Given that 2% of women in the populati<strong>on</strong>have intakes in this range, these women c<strong>on</strong>tribute 2% to the total prevalenceof inadequacy in that populati<strong>on</strong> group. Similarly, those women whoseir<strong>on</strong> intake is in the range 23.6–25.7 mg per day will have a probability of aninadequacy of 0.65. If 20% of the women have intakes in this range, the prevalenceof inadequacy am<strong>on</strong>g women <strong>with</strong> intakes in this range is 20 × 0.65 or13% (Table 7.6). When similar calculati<strong>on</strong>s are performed for each of the otherintake ranges, and then summed, an overall prevalence of inadequacy for thegroup of 66.6% is obtained. In other words, in this example, two thirds of thepopulati<strong>on</strong> of women have intakes that are likely to be below their requirements.Note that such calculati<strong>on</strong>s are easily performed <strong>with</strong> a spreadsheet or a statisticalprogramming language.157
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 7.5Probability of inadequate ir<strong>on</strong> intakes in selected populati<strong>on</strong> subgroups at different ranges of usual intake (mg/day)Probability of Usual intake of children aged Usual intake of children aged Usual intake of females aged Usual intake of menstruatinginadequacy a 1–3 years c<strong>on</strong>suming a diet 4–8 years c<strong>on</strong>suming a diet 14–18 years c<strong>on</strong>suming a diet women c<strong>on</strong>suming a diet fromfrom which the bioavailability from which the bioavailability from which the bioavailability which the bioavailability of ir<strong>on</strong> isof ir<strong>on</strong> is of ir<strong>on</strong> is of ir<strong>on</strong> is5% 10% 15% 5% 10% 15% 5% 10% 15% 5% 10% 15%1.00 21.0aProbability that the requirement for ir<strong>on</strong> is greater than the usual intake. For the purpose of assessing populati<strong>on</strong>s, a probability of 1 has been assignedto usual intakes that are below the 2.5th percentile of requirements, and a probability of 0 has been assigned to usual intakes that fall above the97.5th percentile of requirements. Usual intakes should be adjusted for intra-individual variance as described in secti<strong>on</strong> 7.3.2 (step 1).Source: adapted from reference (91).158
7. DEFINING AND SETTING PROGRAMME GOALTABLE 7.6Prevalence of inadequate ir<strong>on</strong> intakes for menstruating women c<strong>on</strong>suming adiet from which the average ir<strong>on</strong> bioavailability is 5%: an example calculati<strong>on</strong>Probability of Intake range <strong>with</strong> this Proporti<strong>on</strong> of menstruating Prevalence ofinadequacy a probability of women <strong>with</strong> intakes in this inadequacy binadequacy (mg/day) range (%) (%)1.00 63.0 0 0Probability of inadequate intakes for all menstruating women 66.6%abProbability that the requirement for ir<strong>on</strong> is greater than the usual intake. For the purpose ofassessing populati<strong>on</strong>s, a probability of 1 has been assigned to usual intakes that are belowthe 2.5th percentile of requirements, and a probability of 0 has been assigned to usual intakesthat fall above the 97.5th percentile of requirements. Usual intakes should be adjusted forintra-individual variance as described in secti<strong>on</strong> 7.3.2 (step 1).The prevalence of inadequacy = probability of inadequacy for a given intake range × the percentageof women <strong>with</strong> intakes in that range.Having established the prevalence of inadequate intakes, the next step is tosimulate how the distributi<strong>on</strong> of intakes would shift upwards as a result of thec<strong>on</strong>sumpti<strong>on</strong> of ir<strong>on</strong> fortified <strong>food</strong>(s) (in much the same way as was d<strong>on</strong>e insteps 3 and 4 for vitamin A above; see Table 7.4) <strong>with</strong> a view to finding that levelof fortificati<strong>on</strong> that would bring the estimated prevalence of inadequacy downto an acceptable level, say 2–3%.7.3.3.2 IodineBased <strong>on</strong> field experience,WHO has recommended fortificati<strong>on</strong> levels for iodinein salt (283). The current recommendati<strong>on</strong>, designed to provide the adult RNI(i.e. 150µg/day), is to add 20–40 mg iodine/kg salt. This level of fortificati<strong>on</strong>assumes no iodine in the usual diet pre-fortificati<strong>on</strong>, and that the usual amountof salt c<strong>on</strong>sumed is 10 g per day.159
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS7.3.3.3 Folate/folic acidNumerous studies have dem<strong>on</strong>strated that a higher intake of folate by somewomen can reduce their risk of delivering an infant <strong>with</strong> a neural tube defect(see secti<strong>on</strong> 4.2.3). It is generally accepted that women should c<strong>on</strong>sume an additi<strong>on</strong>al400 µg/day as folic acid in fortified <strong>food</strong>s or supplements peric<strong>on</strong>cepti<strong>on</strong>ally(128). Currently, it is unknown whether the reducti<strong>on</strong> in risk resultsfrom the correcti<strong>on</strong> of a folate deficiency or through some other as yet unidentifiedmechanism. However, increasing intake by just 200µg/day through fortificati<strong>on</strong>has been shown to be effective in improving folate status and in loweringthe prevalence of neural tube defects in both Canada (51) and the United States(48,49). Based <strong>on</strong> this evidence, the Pan American Health Organizati<strong>on</strong> has recommendedthat throughout Latin America <strong>food</strong> fortificati<strong>on</strong> interventi<strong>on</strong>sshould provide an additi<strong>on</strong>al 200 µg folic acid per day (339). It is anticipatedthat at these levels of additi<strong>on</strong>al intake, the usual daily intake of folic acid plus<strong>food</strong> folate will exceed the EAR and approach the RNI for the majority of thetarget populati<strong>on</strong>. In any case, it would be appropriate to start <strong>with</strong> the estimati<strong>on</strong>of the nutriti<strong>on</strong>al gap for folate, which may be around that value, and hencethe decisi<strong>on</strong> will have a nutriti<strong>on</strong>al justificati<strong>on</strong>.It should be noted that folate intakes are c<strong>on</strong>venti<strong>on</strong>ally expressed in units ofDietary Folate Equivalents (DFE), where 1µg folate in <strong>food</strong> is equivalent to 1DFE. Because of its higher bioavailability, 1 µg of folic acid actually supplies 1.7DFE and so less folic acid (the synthetic form of folate that is used as a fortificantand in supplements) is required to meet a given requirement for folate(128).7.3.3.4 Vitamin DVitamin D is produced in the skin <strong>on</strong> exposure to ultraviolet light. At latitudesbetween 42 o N and 42 o S, 30 minutes of skin exposure per day (arms and face)is usually sufficient to provide the body <strong>with</strong> all the vitamin D it needs. However,as discussed in secti<strong>on</strong> 4.6, several factors inhibit the ability of the body to synthesizevitamin D and thus increase the risk of vitamin D deficiency. Thesefactors may include living at a more northerly or more southerly latitude (wherethe days are shorter during the winter seas<strong>on</strong>), leaving little skin exposed to ultravioletlight, and having dark skin.Any decisi<strong>on</strong>s about appropriate levels of vitamin D fortificati<strong>on</strong> wouldneed to take exposure to sunlight into account. For instance, in situati<strong>on</strong>swhere exposure to sunlight is adequate but dietary intake of vitamin D is low,it is quite likely that the risk of vitamin D deficiency in a populati<strong>on</strong> will be overestimatedif based <strong>on</strong> intake data al<strong>on</strong>e. For this reas<strong>on</strong>, informati<strong>on</strong> <strong>on</strong> theprevalence of rickets in infants and children, low serum 25-hydroxy vitamin Dc<strong>on</strong>centrati<strong>on</strong>s in the general populati<strong>on</strong>, and osteomalacia and/or osteoporosis160
7. DEFINING AND SETTING PROGRAMME GOALin women, should be evaluated when predicting the level of vitamin D fortificati<strong>on</strong>required.7.3.3.5 NiacinNiacin (vitamin B 3 ) is unique in that it can be synthesized from the amino acid,tryptophan (1 mg niacin can be generated from approximately 60 mg tryptophan).Thus, similar to the situati<strong>on</strong> <strong>with</strong> vitamin D, there will appear to be ahigh prevalence of inadequate intakes of the vitamin using <strong>on</strong>ly the dietaryintake, both before and after fortificati<strong>on</strong>, if n<strong>on</strong>-dietary sources of niacin (i.e.synthesis from tryptophan) are not c<strong>on</strong>sidered. However, because the rate ofniacin synthesis from tryptophan is not known <strong>with</strong> certainty, and probablyvaries <strong>with</strong> life stage and physiological status (e.g. in pregnancy and for younginfants), the most practical approach may be to ignore the c<strong>on</strong>tributi<strong>on</strong> of tryptophanwhen setting fortificati<strong>on</strong> levels. Moreover, the risk of niacin toxicity islow, especially if niacinamide is used as the fortificant (see Table 7.3).As maize c<strong>on</strong>tains niacin in a bound form and is low in tryptophan, populati<strong>on</strong>swhose staple <strong>food</strong> is maize (especially maize that is untreated <strong>with</strong> alkali)are most likely to benefit from niacin fortificati<strong>on</strong> (see secti<strong>on</strong> 4.4.3).7.3.4 Bioavailability c<strong>on</strong>siderati<strong>on</strong>sThe methods used to set EARs already include an adjustment for the bioavailability(i.e. % absorpti<strong>on</strong>) of a nutrient from <strong>food</strong>s, and so when formulatingfortificati<strong>on</strong> levels using the EAR cut-point method there is usually no need tomake any further allowance for this factor. If, however, the bioavailability of thefortificant nutrient is likely to be substantially different from that naturallypresent in the diet, some further adjustment will need to be made.The efficiencyof utilizati<strong>on</strong> of the form of the fortificant nutrient may also need to be c<strong>on</strong>sidered.For example, the c<strong>on</strong>versi<strong>on</strong> rate of synthetic β-carotene to retinol in oilis 2:1, but in the absence of oil, the rate is significantly lower (i.e. 6:1) and theutilizati<strong>on</strong> much less efficient.Micr<strong>on</strong>utrients for which differences in bioavailability may be a factor arelisted in Table 7.7. Electrolytic ir<strong>on</strong>, for instance, is poorly absorbed, and it isrecommended that this particular form of ir<strong>on</strong> fortificant be added at twice thequantity of ferrous sulfate ir<strong>on</strong>, which has a similar bioavailability to n<strong>on</strong>-haemir<strong>on</strong> in the diet (Table 5.2). In c<strong>on</strong>trast, the absorpti<strong>on</strong> of some fortificants, suchas folic acid and vitamin B 12 ,may be substantially higher than their equivalent(i.e. naturally-occurring) forms in <strong>food</strong>s (see secti<strong>on</strong> 7.3.3.3).Ideally, the absorpti<strong>on</strong> of the fortificant nutrient should be c<strong>on</strong>firmed inefficacy trials involving the target populati<strong>on</strong>, especially in situati<strong>on</strong>s wherethere is uncertainty about its bioavailability. If this is not possible, then as aminimum, absorpti<strong>on</strong> data should be obtained from human studies by other161
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 7.7Examples of micr<strong>on</strong>utrients for which the bioavailability of the form used forfortificati<strong>on</strong> differs substantially from their bioavailability in the usual dietNutrient/fortificant compoundIr<strong>on</strong>■ Electrolytic ir<strong>on</strong>■ NaFeEDTA a■ Ferrous bisglycinateVitamin A■ β-carotene bFolate■ Folic acidVitamin B 12Proporti<strong>on</strong> absorbed relative to usual diet0.5 (compared <strong>with</strong> n<strong>on</strong>-haem ir<strong>on</strong> in <strong>food</strong>s)3.0 at high phytate, 1.0 at low phytate (compared<strong>with</strong> n<strong>on</strong>-haem ir<strong>on</strong> in <strong>food</strong>s)2.0–3.0 (compared <strong>with</strong> n<strong>on</strong>-haem ir<strong>on</strong> in <strong>food</strong>s)0.15 from fortified <strong>food</strong>s in the absence of oil,but 0.5 in oil (compared <strong>with</strong> retinol)1.7 (compared <strong>with</strong> Dietary FolateEquivalents of natural <strong>food</strong> folates)2.0 (compared <strong>with</strong> cobalamin in <strong>food</strong>s)abNaFeEDTA, sodium ir<strong>on</strong> ethylenediaminetetraacetic acid.When β-carotene is added as a <strong>food</strong> fortificant, its bioavailability is higher (the c<strong>on</strong>versi<strong>on</strong>factor β-carotene : retinol is 6 : 1 in n<strong>on</strong>-oily <strong>food</strong>s) than that of naturally-occurring β-carotene(in fruits and vegetables), for which the corresp<strong>on</strong>ding c<strong>on</strong>versi<strong>on</strong> factor is 12 : 1.investigators, and its bioavailability evaluated <strong>on</strong>ce the fortificati<strong>on</strong> programmeis in place.7.4 Other factors to c<strong>on</strong>sider when deciding fortificati<strong>on</strong> levelsExperience has shown that in practice, and especially in the case of mass fortificati<strong>on</strong>,the amount of micr<strong>on</strong>utrient that can be added to <strong>food</strong>s is often limitedby various safety, technological and/or ec<strong>on</strong>omic c<strong>on</strong>straints. Of these three limitingfactors, cost c<strong>on</strong>straints tend to be the more flexible, whereas safety andtechnological c<strong>on</strong>straints are more likely to be fixed. However, for somemicr<strong>on</strong>utrients there may be ways of overcoming some of the technological c<strong>on</strong>straints.For instance, in the case of ir<strong>on</strong>, undesirable sensory changes in the<strong>food</strong> vehicle caused by the presence of the fortificant compound might bereduced by using a microencapsulated form of ir<strong>on</strong> instead (see secti<strong>on</strong> 5.1.3.3).Nor are safety c<strong>on</strong>straints necessarily always cast in st<strong>on</strong>e; <strong>with</strong> new knowledgeand improvements in the precisi<strong>on</strong> of ULs, safety c<strong>on</strong>straints may well changeover time.Table 7.8 assesses the strength of each of the three main types of c<strong>on</strong>straintfor the range of micr<strong>on</strong>utrients covered by these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>. The assessment ofthe magnitude of the safety risk is based <strong>on</strong> the closeness of the EAR to the UL;the closer these two values, the greater the risk. The magnitudes indicated in thistable are subjective and are intended <strong>on</strong>ly to highlight those areas that may beof c<strong>on</strong>cern for a given micr<strong>on</strong>utrient.162
7. DEFINING AND SETTING PROGRAMME GOALTABLE 7.8Factors that may limit the amount of fortificants that can be added to a single<strong>food</strong> vehicleNutrient Technological/sensory Safety CostVitamin A X XXX XXX aVitamin D – X XVitamin E – X XXXVitamin C XX X XXX bThiamine (vitamin B 1 ) – – –Riboflavin (vitamin B 2 ) XX – –Niacin (vitamin B 3 ) – XXX c XVitamin B 6 – X –Folic acid – XXX d –Vitamin B 12 – – XIr<strong>on</strong> e XXX XX XZinc XX XXX XCalcium X XX XXX fSelenium – X XIodine X XXX ––, no c<strong>on</strong>straint; X, a minor c<strong>on</strong>straint; XX, moderate c<strong>on</strong>straint; XXX, major c<strong>on</strong>straint.aIf an oil-based form is used to fortify oils or fats, costs can be reduced.bCost c<strong>on</strong>straints are mainly a c<strong>on</strong>sequence of losses during manufacturing, storage, distributi<strong>on</strong>and cooking which mean that a c<strong>on</strong>siderable overage is required.cMuch less of a c<strong>on</strong>cern if niacinamide, as opposed to nicotinic acid, is used as thefortificant.dThe risk of adverse effects is minimized by the co-additi<strong>on</strong> of vitamin B 12 .eRefers to the more bioavailable forms.fCost c<strong>on</strong>straints are mainly a c<strong>on</strong>sequence of the need to add such large amounts.7.4.1 Safety limitsThe safety of fortificati<strong>on</strong> can be assessed by comparing predicted micr<strong>on</strong>utrientintakes (in particular the intakes that will occur at higher levels of fortificati<strong>on</strong>and at higher intakes of fortified <strong>food</strong>s, calculated as described in secti<strong>on</strong>7.3.2) <strong>with</strong> the UL (Table 7.3). Even if a micr<strong>on</strong>utrient has no recommendedUL, high levels of micr<strong>on</strong>utrient additi<strong>on</strong>s should be avoided, especially if thereis no evidence of derived benefit from levels of intake in excess of the RNI.7.4.2 Technological limitsThe technological limit is defined as the highest possible level of micr<strong>on</strong>utrientadditi<strong>on</strong> that does not cause adverse organoleptic changes in the <strong>food</strong> vehicle.The effects of added micr<strong>on</strong>utrients <strong>on</strong> the organoleptic properties of the chosen<strong>food</strong> vehicle must be tested at an early stage, and alternative forms of the fortificantused if necessary (see Part III). Technological incompatibility is usually163
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSless of a c<strong>on</strong>straint in the case of <strong>food</strong> products fortified through targeted ormarket-driven interventi<strong>on</strong>s; such products tend to be distributed to c<strong>on</strong>sumersin specialized individual packages, and as final products (see secti<strong>on</strong> 7.5.2).7.4.3 Cost limitsThe cost limit is defined as the highest increase in the cost of the <strong>food</strong> due tofortificati<strong>on</strong> that is acceptable to producers and c<strong>on</strong>sumers. Indeed, <strong>on</strong>e of themost important criteria for a successful and sustainable <strong>food</strong> fortificati<strong>on</strong> programmein free trade ec<strong>on</strong>omies is a low proporti<strong>on</strong>al increase in product priceas a result of fortificati<strong>on</strong>. This is especially true of mass fortified products. Theissue of cost is usually less of a c<strong>on</strong>straint in the case of targeted and marketdrivenprocessed <strong>food</strong>s, as the price of the product tends to be sufficiently highto absorb the costs associated <strong>with</strong> fortificati<strong>on</strong>.Table 7.9 shows the annual investment required to provide an adult male <strong>with</strong>100% of his EAR of 14 essential micr<strong>on</strong>utrients, taking into account micr<strong>on</strong>utrientlosses during producti<strong>on</strong>, distributi<strong>on</strong> and storage as well as duringcooking, and also any variability in the fortificati<strong>on</strong> process which might lead toa lowering of the amount of fortificant delivered (e.g. uneven mixing). The totalcost for a dry n<strong>on</strong>-oily <strong>food</strong>, such as wheat flour, is approximately US$ 4.00 peryear, or US$ 0.01 per day. According to these calculati<strong>on</strong>s, the most expensivefortificants are calcium (because larger amounts are needed), vitamin A, vitaminE and vitamin C (because of overage needed to compensate for losses). Thecheapest – each costing less than US$ 0.02 per year – are thiamine (vitamin B 1 ),vitamin B 6 , folic acid, zinc and iodine.The above cost estimates apply to the large-scale centralized fortificati<strong>on</strong> ofa staple, i.e. fortificati<strong>on</strong> that is carried out in just a few large industrial units.Under such circumstances, the purchase price of the micr<strong>on</strong>utrients accountsfor by far the greatest proporti<strong>on</strong> (at least 80–90%) of the total fortificati<strong>on</strong> cost.When fortificati<strong>on</strong> is carried out by multiple, smaller-scale enterprises, both theinitial investment costs (e.g. of equipment) and the running costs (e.g. of qualityc<strong>on</strong>trol procedures) are proporti<strong>on</strong>ally higher, a factor which might hinder thefeasibility and sustainability of the programme. Such c<strong>on</strong>siderati<strong>on</strong>s not<strong>with</strong>standing,in many settings <strong>food</strong> fortificati<strong>on</strong> can be a very affordable way of correctinginadequate micr<strong>on</strong>utrient intakes, and more often than not, the mainchallenge is finding a suitable industrially-manufactured <strong>food</strong> vehicle that is c<strong>on</strong>sumedin sufficient amounts by the populati<strong>on</strong> at risk.7.5 Adapting the EAR cut-point methodology to mass, targetedand market-driven fortificati<strong>on</strong> interventi<strong>on</strong>sThe EAR cut-point method can be used to select appropriate fortificati<strong>on</strong> levelsand to estimate their impact <strong>on</strong> the prevalence of inadequate intakes for all three164
7. DEFINING AND SETTING PROGRAMME GOALTABLE 7.9Estimated costs of selected fortificants aVitamin AAdult Nutrient Cost of Overage b Annual costEAR c<strong>on</strong>tent of fortificant (%) of fortificantfortificant (US$/kg) (US$) c(%)■ Vitamin A (SD-250) 429 µg 7.5 42 50 0.136■ Vitamin A palmitate, 429 µg 30 52 30 0.0421 milli<strong>on</strong> IUVitamin D■ Watersoluble 5 µg 0.25 33 20 0.035■ In oil, 1 milli<strong>on</strong> IU/g 5 µg 2.5 80 20 0.008Vitamin E 8 mg 67 26 20 0.163Vitamin C 37 mg 100 10 250 d 0.567Thiamine (vitamin B 1 ) 1.0 mg 81 24 40 0.018Riboflavin (vitamin B 2 ) 1.1 mg 100 38 30 0.024Niacin (vitamin B 3 ) 12mg 99 9 10 0.053Vitamin B 6 1.1 mg 82 28 20 0.020Folic acid 188 µg e 100 90 50 0.011Vitamin B 12 , 0.1% watersoluble 2.0 µg 0.1 38 30 0.043Ir<strong>on</strong> f■ NaFeEDTA 7.0 mg 13 15.45 5 0.383■ Ferrous bisglycinate 7.0 mg 20 25 5 0.402■ Ferrous fumarate 10.5 mg 33 5.12 5 0.075■ FeSO 4, dried 10.5 mg 33 2.35 5 0.034■ FeSO 4 , encapsulated 10.5 mg 16 12.28 5 0.371■ Electrolytic ir<strong>on</strong> 21.1 mg 97 5.76 5 0.058Zinc (as oxide) 6 mg g 80 3.35 5 0.012Calcium (as phosphate) 833 mg 39 2.7 5 2.652Iodine (as potassium iodate) 107 µg 59 20 25 0.002NaFeEDTA, sodium ir<strong>on</strong> ethylenediaminetetraaectic acid; FeSO 4 , ferrous.aThe cost of supplying enough micr<strong>on</strong>utrient to meet 100% of the EAR of an adult male, dailyfor <strong>on</strong>e year (via dry <strong>food</strong>).bThe overage is an additi<strong>on</strong>al amount that must be added to compensate for losses duringproducti<strong>on</strong>, storage, <strong>food</strong> producti<strong>on</strong> and distributi<strong>on</strong>.cIncludes an overage of +20% to cover variability in the fortificati<strong>on</strong> process.dVitamin C is <strong>on</strong>e of the least stable fortificants and a high overage is normally required. If,however, the fortified <strong>food</strong> is not subject to heat or oxidati<strong>on</strong>, the overage can be much lower.eAs folic acid is 1.7 times more bioavailable than naturally-occurring <strong>food</strong> folates, the EAR forfolate has been divided by 1.7.fThe EAR for ir<strong>on</strong> depends <strong>on</strong> its bioavailability from the diet as well as the identity of the ir<strong>on</strong>compound used as the fortificant. The values given here refer to white wheat flour (low extracti<strong>on</strong>),and apply to diets <strong>with</strong> similar bioavailabilities. If the diet c<strong>on</strong>tains large amounts of ir<strong>on</strong>absorpti<strong>on</strong> inhibitors, the EAR should be multiplied by a factor of around 2. Reduced ir<strong>on</strong> isnot included; its absorpti<strong>on</strong> would be at most about half that of electrolytic ir<strong>on</strong>.gAssuming a moderate bioavailability of zinc.165
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTStypes of interventi<strong>on</strong> – mass, targeted and market-driven. However, in each case,there are some unique issues that need to be addressed; these are outlined below.7.5.1 Mass fortificati<strong>on</strong>7.5.1.1 Setting levels of micr<strong>on</strong>utrient additi<strong>on</strong>sMany industrialized and some developing countries have a l<strong>on</strong>g history of experience<strong>with</strong> mass fortificati<strong>on</strong>. In many cases, fortificati<strong>on</strong> levels were selectedempirically, that is to say, were based <strong>on</strong> a combinati<strong>on</strong> of experience elsewhereand technological and cost c<strong>on</strong>straints, rather than <strong>on</strong> any attempt to derive,in a systematic fashi<strong>on</strong>, fortificati<strong>on</strong> levels likely to provide the most benefit.This was especially true of situati<strong>on</strong>s and settings where <strong>food</strong> intake datawere not available, yet there was a str<strong>on</strong>g desire to move ahead <strong>with</strong> fortificati<strong>on</strong>.However, unless <strong>food</strong> and nutrient intake data are used as the basis ofprogramme design and evaluati<strong>on</strong> – as described earlier in this chapter – itcannot be known whether those at greatest risk of deficiency in a specific nutrient(and who have the lowest pre-fortificati<strong>on</strong> intakes) will c<strong>on</strong>sume enough ofthe fortified <strong>food</strong> to improve their nutrient intake significantly, and whether thosewho have the highest pre-fortificati<strong>on</strong> intakes will be at risk of excessive intakesafter fortificati<strong>on</strong>.Having emphasized the importance of a more rigorous approach to settingfortificati<strong>on</strong> levels, it is nevertheless useful to know what fortificati<strong>on</strong> levels arealready in use for specific <strong>food</strong>s in other locati<strong>on</strong>s. At the very least, knowledgeof what levels are used elsewhere will provide some guidance as to what levelsof fortificati<strong>on</strong> are technologically and ec<strong>on</strong>omically feasible in which <strong>food</strong>s.Interestingly, as can be seen from Table 7.10, the band of currently used fortificati<strong>on</strong>levels for each type of <strong>food</strong> is relatively narrow. It must be stressed thatthe impact of fortificati<strong>on</strong> at these levels <strong>on</strong> nutrient intake and nutriti<strong>on</strong>al statushas <strong>on</strong>ly been adequately evaluated in a handful of settings and that these levelscannot be recommended universally. Furthermore, fortificati<strong>on</strong> levels in use in<strong>on</strong>e locati<strong>on</strong> may be inappropriate in another, and should not be used <strong>with</strong>outc<strong>on</strong>firming their suitability by using the EAR and UL cut-point method, asdescribed in these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>.7.5.1.2 C<strong>on</strong>straintsIn the case of mass fortificati<strong>on</strong> programmes, which tend to rely <strong>on</strong> staples andc<strong>on</strong>diments as the <strong>food</strong> vehicle, cost is often the most significant limiting factor.Staples and c<strong>on</strong>diments are c<strong>on</strong>sumed frequently and in large amounts, not <strong>on</strong>lyby the populati<strong>on</strong> directly but also by <strong>food</strong> industries. Even small variati<strong>on</strong>s inprice can thus have profound c<strong>on</strong>sequences; oppositi<strong>on</strong> to fortified products <strong>on</strong>the grounds of cost, can, for example, lead to an increase in deceptive practices,even smuggling.166
7. DEFINING AND SETTING PROGRAMME GOALTABLE 7.10Examples of levels of micr<strong>on</strong>utrients currently added to staples and c<strong>on</strong>diments worldwide (mg/kg)Nutrient Milk Evaporated Powdered Margarine Vegetable Sugar Wheat Pasta Corn Pre–cooked Maize Maize Soy/fish Saltmilk milk oil flour masa maize flour meal Sauceflour flourVitamin A 0.7–1.0 2–3 4.5–7.5 5–15 5–15 5–15 1–5 – – 2.8 1–2 – –Vitamin D 0.01 0.01 0.05–0.06 0.02–0.15 – – 0.014 – – – – – – –Vitamin E – – – – – – – – – – – – – –Vitamin C – – – – – – – – – – – – – –Thiamine – – – – – – 1.5–7.0 8–10 1–6 3.1 2.4 2–3 – –(vitamin B1)Riboflavin – – – 16 – – 1–5 3–5 1–5 2.5 1.7–2.5 – –(vitamin B2)Niacin – – – 180 – – 15–55 35–57 25–50 51 1.6 19–30 – –(vitamin B3)Vitamin B6 – – – 20 – – 2.5 – – – – 2–3 – –Folic acid 2 – – 0.5–3.0 – 0.5–3.0 – – 0.4–0.5 – –Vitamin B12 – – – – – – 0.01 a – – – – – – –Ir<strong>on</strong> b— NaFeEDTA – – – – – – – – – – – – 250 500 c— Ferrous – – – – – – – – 22 – – – – –— bisglycinate— Ferrous – – – – – – 30–45 30 30 30+ – – – –sulfate orfumarate— Electrolytic – – – – – – 45–60 25–35 30–60 20+ 9–14 – –ir<strong>on</strong>Zinc (oxide) – – – – – – 15–30 – 15–30 – – – – –Calcium – – – – – – 2 100–3 900 – – – – – – –Iodine – – – – – – – – – – – – – 15–60NaFeEDTA, sodium ir<strong>on</strong> ethylenediaminetetraacetic acid.As recommended at a recent PAHO/WHO meeting (339).Usually <strong>food</strong>s are fortified <strong>with</strong> <strong>on</strong>ly <strong>on</strong>e ir<strong>on</strong> compound, but in the case of pre-cooked maize flour trials are currently underway to assess the viability of using more than<strong>on</strong>e ir<strong>on</strong> fortificant.abAs encapsulated ferrous sulfate, but to date this has <strong>on</strong>ly been used <strong>on</strong>ly in experimental trials.c167
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSThe cost of fortifying <strong>on</strong>e t<strong>on</strong>ne of wheat flour <strong>with</strong> ferrous fumarate (45 mgFe/kg), zinc oxide (30 mg/kg), thiamine (6.5 mg/kg), riboflavin (4 mg/kg), niacin(50 mg/kg), folic acid (2.0 mg/kg), vitamin B 12 (0.010 mg/kg) and vitamin A (2.0mg/kg) has been estimated to be US$ 5. At a per capita c<strong>on</strong>sumpti<strong>on</strong> of 100 gof wheat flour a day, this is equivalent to <strong>on</strong>ly US$ 0.182 per year or US$ 0.0005per day per pers<strong>on</strong>. Nevertheless, in a country <strong>with</strong> 10 milli<strong>on</strong> c<strong>on</strong>sumers, thefortificati<strong>on</strong> costs amount to some US$ 5000 per day (US$ 1.825 milli<strong>on</strong> peryear). At this cost level, some manufacturers might well be tempted to take thatas additi<strong>on</strong>al profit rather than add the micr<strong>on</strong>utrients.The cost of fortifying wheat flour <strong>with</strong> the same micr<strong>on</strong>utrients listed abovemight increase the price of the flour by US$ 0.30–0.50 per kg, or by 1.0–1.7%relative to that of the unfortified product. Although such increments can beincorporated into the product price, in a free-market ec<strong>on</strong>omy even small pricedifferentials might be too much to preserve the market share of the fortifiedproduct against a n<strong>on</strong>-fortified alternative, especially if the competitive rules arenot equal for all. For example, fortificati<strong>on</strong> of salt <strong>with</strong> 20–40 mg iodine/kg usingpotassium iodate costs US$ 1.25 per t<strong>on</strong>ne, or US$ 0.0000125 per day perpers<strong>on</strong> (US$ 0.005 per year per pers<strong>on</strong>) assuming a daily c<strong>on</strong>sumpti<strong>on</strong> of 10 gof salt. This equates to around US$ 45 625 per year for a country of 10 milli<strong>on</strong>pers<strong>on</strong>s.Thus, although the absolute annual investment is relatively low, this representsan increment in price of 2% over raw salt (assuming the price of salt isUS$ 0.06 per kg). Even this modest price increment is disliked by many smallproducers who fear loss of their market share to the extent that they would avoidadding iodine if not for strict governmental enforcement. Herein lies the reas<strong>on</strong>why salt is currently not more widely used as a vehicle for other micr<strong>on</strong>utrients,even if their additi<strong>on</strong> were technically feasible and biologically efficacious. If,however, mechanisms to overcome impediments of this nature, such as subsidiesor effective and reliable enforcement mechanisms, were to be devised, saltmay yet become a more attractive opti<strong>on</strong> as a vehicle for mass fortificati<strong>on</strong>. Saltcould also be used in targeted programmes, where price increases due to thecost of fortificati<strong>on</strong> tend to be less limiting.In summary, experience dictates that a mass fortificati<strong>on</strong> in an open marketec<strong>on</strong>omy works best when the increase in the price of the fortified product, relativeto the unfortified versi<strong>on</strong>, does not exceed 1–2%. However, this is by nomeans a universal prescripti<strong>on</strong> and any decisi<strong>on</strong>s relating to cost limits can <strong>on</strong>lybe taken by government and industry depending <strong>on</strong> the situati<strong>on</strong> in any givencountry.Annex D describes a procedure that can be used for setting Feasible Fortificati<strong>on</strong>Levels for mass fortificati<strong>on</strong> programmes based <strong>on</strong> c<strong>on</strong>siderati<strong>on</strong> ofsafety, technological and cost c<strong>on</strong>straints. If the combined effect of these c<strong>on</strong>straintsis to reduce the amount of nutrient that can be added to below thatwhich is required to achieve a given intake goal, then more than <strong>on</strong>e <strong>food</strong> may168
7. DEFINING AND SETTING PROGRAMME GOALneed to be fortified or some nutrients may have to be supplied through otherstrategies, such as supplementati<strong>on</strong>.7.5.2 Targeted fortificati<strong>on</strong>With targeted fortificati<strong>on</strong>, the various c<strong>on</strong>straints <strong>on</strong> micr<strong>on</strong>utrient additi<strong>on</strong>sare generally less restrictive than those that operate in the case of <strong>food</strong> productssubject to mass fortificati<strong>on</strong>. Not <strong>on</strong>ly is the target group more clearly defined(refugees, displaced pers<strong>on</strong>s and young children are usually the main beneficiaries),but the fortified <strong>food</strong>s are usually in their final form or are offered indefined serving sizes, so that the risk of exceeding ULs is reduced. In additi<strong>on</strong>,the presentati<strong>on</strong> and sensory properties of <strong>food</strong>s selected for targeted fortificati<strong>on</strong>are such that any changes introduced by the additi<strong>on</strong> of micr<strong>on</strong>utrients aremore easily hidden, and the cost of fortificati<strong>on</strong> is usually compatible <strong>with</strong> theprice of the product or partly borne by the financial supporters of the programme.Nevertheless, it is always instructive to assess technological compatibilityand overall cost of targeted fortified <strong>food</strong>s at the programme planningstage.7.5.2.1 Blended <strong>food</strong>s<str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> covering the fortificati<strong>on</strong> of blended <strong>food</strong>s for refugees and displacedpers<strong>on</strong>s are available elsewhere (62) and thus are not discussed in detail here.7.5.2.2 Complementary <strong>food</strong>sThat the micr<strong>on</strong>utrient c<strong>on</strong>tent of breast milk may be c<strong>on</strong>siderably lower inundernourished women is a c<strong>on</strong>cern in several parts of the world. Nutrientsmost likely to be reduced in the breast milk of undernourished mothers includevitamin A, all B vitamins except folate, iodine and selenium. The fortificati<strong>on</strong> ofcomplementary <strong>food</strong>s thus provides a means of supplying additi<strong>on</strong>al nutrientsto infants and children who are still receiving breast milk. However, becauseinfants and young children also require their diets to have a high nutrient density(i.e. a high c<strong>on</strong>centrati<strong>on</strong> of nutrient per kcal), in some settings it may be difficultto achieve a low risk of inadequate nutrient intakes even when complementary<strong>food</strong>s are fortified.The process of setting the level of fortificati<strong>on</strong> for complementary <strong>food</strong>s issimilar to that described earlier in this chapter (see secti<strong>on</strong> 7.3.2). The startingpoint is again the distributi<strong>on</strong> of usual intakes, although in this case is it necessaryto c<strong>on</strong>sider the intakes from both breast milk and complementary <strong>food</strong>s foreach nutrient being studied. Breast-milk intakes can be estimated from publishedinformati<strong>on</strong> <strong>on</strong> the compositi<strong>on</strong> of breast milk (such as that published by WHO,which includes data from both industrialized and developing countries (340),169
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSand intakes from other sources can be collected using 24-hour recall methodsfor a representative sample of the group of interest.Having obtained the relevant intake data, the procedure for setting fortificati<strong>on</strong>levels is then much the same as previously, the steps being as follows:Step 1. Determine the prevalence of intakes that are below the EARFirst, review the intake distributi<strong>on</strong>s and determine the prevalence of intakes thatare below the EAR; decide if this level of inadequate intakes is acceptable. If itis not, then fortificati<strong>on</strong> of complementary <strong>food</strong>s could be c<strong>on</strong>sidered.Step 2. Decide what level of prevalence of inadequate intakesis acceptableNext, decide <strong>on</strong> an acceptable prevalence of inadequacy (i.e. the percentage ofthe children <strong>with</strong> intakes below the EAR). Often 2–3% is taken to be themaximum desirable prevalence of inadequate intakes. Then determine the levelof fortificati<strong>on</strong> that will move the prevalence of inadequacy down to this acceptablelevel.Step 3. Select a <strong>food</strong> vehicleChoose the most appropriate vehicle for fortificati<strong>on</strong>, i.e. the <strong>on</strong>e that will reachmost of the children, or will reach those <strong>with</strong> the greatest need.Step 4. Simulate the impact of fortificati<strong>on</strong>Finally, calculate through simulati<strong>on</strong> the likely impact of fortificati<strong>on</strong> of thechosen <strong>food</strong> vehicle <strong>on</strong> the prevalence of inadequate intakes and proporti<strong>on</strong> ofintakes that are in excess of the UL.With the excepti<strong>on</strong> of ir<strong>on</strong> and zinc, EARs are not defined for infants aged0–12 m<strong>on</strong>ths. For this age group, recommended intakes are expressed in termsof Adequate Intakes (AIs), in which case the nutriti<strong>on</strong>al goal of a fortificati<strong>on</strong> programmewould be the raising of the mean intake of the target group to the AI.Simpler alternative approaches to setting fortificati<strong>on</strong> levels for complementary<strong>food</strong>s do, however, exist. One opti<strong>on</strong> is to estimate the size of the gapbetween the usual median intake and the recommended intake (either the EARor the AI, depending <strong>on</strong> the nutrient of interest); this then equates to the amountof micr<strong>on</strong>utrient that needs to be added in order to achieve the desired nutriti<strong>on</strong>algoal. Dividing the nutriti<strong>on</strong>al gap by the daily amount of <strong>food</strong> c<strong>on</strong>sumedgives the amount of micr<strong>on</strong>utrient that needs to be supplied per gram of thefortified <strong>food</strong> 1 .1The fortificati<strong>on</strong> level is more c<strong>on</strong>venti<strong>on</strong>ally expressed as an amount per 100 g or per servingsize (i.e. the amount per gram multiplied by the average serving size, usually 40 g).170
7. DEFINING AND SETTING PROGRAMME GOALThe other opti<strong>on</strong>, which is the easier of the two because there is no need forintake data, is to simply add a specific proporti<strong>on</strong> of the EAR (or AI) of thetarget group in the hope that by so doing the nutriti<strong>on</strong>al needs of the majorityof the children will be met. Again it is necessary to know how much of a givencomplementary <strong>food</strong> is c<strong>on</strong>sumed per day, and also what the usual serving sizesare in order to derive a fortificati<strong>on</strong> level per 100 g of product or per servingsize.The Codex Alimentarius Commissi<strong>on</strong> provides recommendati<strong>on</strong>s for thecompositi<strong>on</strong> of certain <strong>food</strong>s designated for infants and young children. Theseare subject to a process of c<strong>on</strong>tinual review and are regularly revised. In the caseof supplementary <strong>food</strong>s for older infants and young children, when the <strong>food</strong> issupplemented <strong>with</strong> specific nutrients, the Codex recommendati<strong>on</strong> is to add atleast two thirds of the reference daily requirement per 100 g of <strong>food</strong> (341). Inpractice, this means that if an average serving size for this age group is around40 g, each serving should provide between 30% and 50% of the EAR in orderto satisfy the daily nutrient needs in 2 to 3 servings a day. Obviously, if detaileddietary informati<strong>on</strong> is available, the micr<strong>on</strong>utrient c<strong>on</strong>tent of complementary<strong>food</strong>s might be adjusted to the exact characteristics and needs of the targetgroup.7.5.3 Market-driven fortificati<strong>on</strong>Food manufacturers add micr<strong>on</strong>utrients to their products not just to increasetheir nutriti<strong>on</strong>al value but also to increase their appeal to the health c<strong>on</strong>sciousc<strong>on</strong>sumer. This business-oriented initiative can play a positive role in publichealth by improving the supply of essential nutrients that are sometimes difficultto provide in sufficient amounts via mass fortificati<strong>on</strong>. So far, the publichealth impact of fortificati<strong>on</strong> of market-driven processed <strong>food</strong>s has been verymodest in developing countries but its importance is expected to be greater inthe future, largely as a natural c<strong>on</strong>sequence of increasing urbanizati<strong>on</strong> and availabilityof such <strong>food</strong>s.The main aim of regulating the level of fortificants in processed <strong>food</strong>s is preservingthe nutriti<strong>on</strong>al balance and safety of the diet for the populati<strong>on</strong> at large.To this end, minimum levels need to be set to ensure that reas<strong>on</strong>able amountsof micr<strong>on</strong>utrients are added to <strong>food</strong> products; these must be stated <strong>on</strong> theproduct label, and may be referred to when advertising the product. It is importantto also fix maximum levels so as to reduce the risk of an excessive nutrientintake through the c<strong>on</strong>sumpti<strong>on</strong> of fortified <strong>food</strong>s, especially for those micr<strong>on</strong>utrients<strong>with</strong> well-established UL values (see Chapter 11). It may also be desirableto regulate which <strong>food</strong>s can be fortified (see secti<strong>on</strong> 7.5.3.3).171
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS7.5.3.1 Nutriti<strong>on</strong>al Reference Values (NRVs)<str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> nutriti<strong>on</strong> labelling that are applicable to all <strong>food</strong>s including fortified<strong>food</strong>s have been produced by the Codex Alimentarius Commissi<strong>on</strong> (342).In an attempt to harm<strong>on</strong>ize the labelling of <strong>food</strong>s <strong>with</strong> respect to their nutrientc<strong>on</strong>tents, the Codex guidelines define a set of Nutrient Reference Values(NRVs), which are based <strong>on</strong> the FAO/WHO RNI values for adult males, as areference for the general populaiti<strong>on</strong>. Unlike RNIs, NRVs are not given for specificage or physiological groups but are designed to apply to all family membersaged over 3 years. The current NRVs (see Table 7.11) are based <strong>on</strong> the 1996FAO/WHO RNI values (210), and will be adjusted in accordance <strong>with</strong> the morerecent RNI values published by the FAO/WHO (93).TABLE 7.11Codex Nutrient Reference Values (NRVs) for selected micr<strong>on</strong>utrientsNutrient Codex NRV a FAO/WHO RNI for adult males bCalcium (mg) 800 1 000Iodine (µg) 150 150Ir<strong>on</strong> (mg) 14 13.7Magnesium (mg) 300 260Selenium (µg) – 34Zinc (mg) 15 7Biotin (µg) – 30Vitamin B 6 (mg) 2 1.3Folate b (µg DFE) 200 400Vitamin B 12 (µg) 1 2.4Niacin (vitamin B 3 ) (mg) 18 16Riboflavin (vitamin B 2 ) (mg) 1.6 1.3Thiamine (vitamin B 1 ) (mg) 1.4 1.2Vitamin C (mg) 60 45Vitamin A d (µg RE) 800 600Vitamin D e (µg) 5 5Vitamin E (α–tocopherol) (mg) – 10.0aThe Nutrient Reference Value (NRV) is a dietary reference value defined by the Codex AlimentariusCommissi<strong>on</strong> for the purposes of harm<strong>on</strong>izing the nutriti<strong>on</strong> labeling of processed<strong>food</strong>s and used as a reference for the general populati<strong>on</strong> (342).bThe FAO/WHO RNIs listed here are those published in 1996 (210), some of which have sincebeen revised.c1 DFE = Dietary folate equivalent = 1 µg <strong>food</strong> folate = 0.6 µg folic acid from fortified <strong>food</strong>s,which means that 1 µg folic acid = 1.7 DFE.dRE = retinol equivalents (1 µg RE = 3.33 IU vitamin A).eAs calciferol (1 µg calciferol = 40 IU vitamin D).Sources: adapted from references (210,342).172
7. DEFINING AND SETTING PROGRAMME GOAL7.5.3.2 Setting safe maximum limits for market-driven fortificati<strong>on</strong> ofprocessed <strong>food</strong>sThe fact that market-driven fortified processed <strong>food</strong>s are usually marketed toall family members, rather than to specific age or physiological groups, presentsa number of difficulties in terms of setting maximum limits <strong>on</strong> the permittedlevels of fortificants in such <strong>food</strong>s. The difficulties are compounded by the factthat the same serving size of the fortified <strong>food</strong> (breakfast cereals, beverages andnutriti<strong>on</strong>al bars, for example) is comm<strong>on</strong> to all members of the family. Theproblem therefore arises that by using maximum limits that are based <strong>on</strong> theNRVs (i.e. RNIs of adult males; see secti<strong>on</strong> 7.5.3.1), unnecessarily largeamounts of micr<strong>on</strong>utrients may be delivered to children by fortified <strong>food</strong>s. Inthis c<strong>on</strong>text, it is worth noting that, for some micr<strong>on</strong>utrients (vitamin A, niacinas nicotinic acid, folate, zinc, calcium and iodine), the UL for children below 8years of age is very close to the EAR for adult males (see Tables 7.2 and 7.3).Establishing maximum levels for nutrient additi<strong>on</strong>s that take into account theabove safety c<strong>on</strong>cerns thus requires adopting some form of risk assessmentappraisal. Such approaches base the calculati<strong>on</strong> of a safe maximum limit <strong>on</strong>accepted values of the UL for the most vulnerable groups, which in this caseare children in the age group 4–8 years. Then, assuming that the amounts ofmicr<strong>on</strong>utrients provided by the diet and via <strong>on</strong>going mass fortificati<strong>on</strong> programmesare known, the maximum micr<strong>on</strong>utrient c<strong>on</strong>tent per serving size of amarket-driven fortified processed <strong>food</strong> is given by the following equati<strong>on</strong> (a):In order to apply this equati<strong>on</strong>, it is necessary to estimate the number of servingsof processed <strong>food</strong>s that are c<strong>on</strong>sumed. This can d<strong>on</strong>e as follows:The usual serving size for solid <strong>food</strong>s is generally assumed to be 50 g and thatfor beverages, 250 ml after rec<strong>on</strong>stituti<strong>on</strong> to liquid. However, for the purposesof this derivati<strong>on</strong> it is better to define the serving size in terms of energy (i.e. inkcal) in order to preserve the nutriti<strong>on</strong>al balance of the diet. Table 7.12 summarizesthe usual energy densities of a variety of commercially-available <strong>food</strong>s,from which it can be seen that the smallest dietary serving size is 40 kcal. Thus,a serving of solid <strong>food</strong>s (50 g) c<strong>on</strong>tains 5 dietary servings, a serving of milk orcereal-based beverages, 6 dietary servings, and sugar-based beverages, 1 dietaryserving.173
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 7.12Energy densities of comm<strong>on</strong> <strong>food</strong> presentati<strong>on</strong>sFood presentati<strong>on</strong> Usual serving Energy density Energy density per 100 gsize per serving or 100 mlSolid 50 g 160 kcal 320 kcalMilk or cereal-based 250 ml 200 kcal 80 kcalbeveragesSugar-based beverages 250 ml 100 kcal 40 kcalIf it is assumed that 30% of an individual’s daily energy intake (2000 kcal) isderived from fortified processed <strong>food</strong>s, the amount of energy provided by these<strong>food</strong>s would be:2000 kcal × 0.3 = 600kcal.In terms of the number of the smallest dietary serving size, expressed as anenergy density (i.e. 40 kcal), this amount of energy equates to:600kcal/40kcal = 15 servings.Thus, the previous equati<strong>on</strong> can be transformed as follows:Box 7.3 illustrates the use of this procedure for milk and sugar-based beverages.Under normal circumstances, and after c<strong>on</strong>sidering nutrient amounts suppliedby the diet, it is unlikely that the maximum safe limits per usual servingsize for the nutrients menti<strong>on</strong>ed in Table 7.13 (<strong>with</strong> the excepti<strong>on</strong> of calcium)will be in excess of 30% of the RNI in the case of solid <strong>food</strong>s and milk- or cerealbasedbeverages and in excess of 15% of the RNI in the case of sugar-basedbeverages.7.5.3.3 Keeping the nutriti<strong>on</strong>al balanceSome micr<strong>on</strong>utrients were intenti<strong>on</strong>ally omitted from the discussi<strong>on</strong> in the precedingsecti<strong>on</strong>, because either they do not have a recognized UL (health risks174
7. DEFINING AND SETTING PROGRAMME GOALBOX 7.3Example: setting maximum safe levels for the fort i ficati<strong>on</strong> ofmilk and sugar-based beverages <strong>with</strong> vitamin A1. MilkA milk beverage is to be fortified <strong>with</strong> vitamin A. The fortified product is aimedat a populati<strong>on</strong> in which the daily intake of retinol (preformed vitamin A) throughthe diet and from <strong>on</strong>going mass fortificati<strong>on</strong> programmes by children is approximately300 µg.Given that the UL for vitamin A in children aged 4–8 years is 900 µg (Table 7.3).the maximum c<strong>on</strong>tent of vitamin A per 40 kcal serving size will be:(900 − 300 µg vitamin A)/15 servings = 40 µg vitamin A/serving.By using the relevant c<strong>on</strong>versi<strong>on</strong> factor (Table 7.14), we can calculate themaximum safe vitamin A c<strong>on</strong>tent for a 250 ml serving of milk, as follows:40 µg vitamin A × 5.0 = 200 µg vitamin A.Expressed as a percentage of the adult male RNI (see Table 7.1), the maximumvitamin A c<strong>on</strong>tent of a 250 ml serving of the fortified milk is:200/600 × 100 = 33%,and expressed as a percentage of the current NRV (see Table 7.11), themaximum vitamin A c<strong>on</strong>tent of a the same sized serving of milk is:200/800 × 100 = 25%.2. Sugar-based beveragesA similar calculati<strong>on</strong> for a sugar-based beverage yields a maximum vitamin Ac<strong>on</strong>tent of 100 µg (i.e. 40 µg × 2.5) per 250 ml of beverage (or rec<strong>on</strong>stitutedpowder), which represents 17% of the adult male RNI and 12.5% of the currentNRV for this vitamin.have not, as yet, been identified), or their UL is high enough to not to raiseserious c<strong>on</strong>cerns about the safety of high intakes from fortified <strong>food</strong>s. However,in the interests of maintaining an adequate balance in the diet, it is recommendedthat these other nutrients be added to processed fortified <strong>food</strong>s in roughly thesame proporti<strong>on</strong> as those micr<strong>on</strong>utrients for which large intakes are undesirable.In practice this means limiting micr<strong>on</strong>utrient additi<strong>on</strong>s to between 15% and 30%of the adult RNI in the case of solid <strong>food</strong>s and milk- or cereal-based beverages,and to half of these values (i.e. 7.5–15%) in the case of sugar-based beverages.175
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 7.13Calculated maximum micr<strong>on</strong>utrient c<strong>on</strong>tent a per 40 kcal-sized serving,assuming no other sources of micr<strong>on</strong>utrient in the dietNutrient b UL (children aged Maximum amount4–8 years)Per 40 kcal serving As a % of the RNI cVitamin A (as retinol) (µg RE) 900 µg 60µg 10Niacin (as nicotinic acid d ) (mg) 15 mg 1.0 mg 6Folic acid (mg) 400 µg 27µg 7Ir<strong>on</strong> (mg) 40 mg 3 mg 22Zinc (mg) 12 mg 0.6 mg 4Calcium (mg) 2 500 mg 167 mg 17Iodine (µg) 300 µg 20µg 13UL, Tolerable Upper Intake Limit; RNI, Recommended Nutrient Intake.aMaximum levels listed here should be reduced by an amount proporti<strong>on</strong>al to the amount ofnutrient supplied by the diet (including though mandatory mass fortificati<strong>on</strong> programmes).bThere are other micr<strong>on</strong>utrients <strong>with</strong> UL values, but they are not included here because it wouldbe very difficult to approach the UL through the c<strong>on</strong>sumpti<strong>on</strong> of fortified <strong>food</strong>s.cAs a percentage of the RNI for adult males.dNiacinamide can be used <strong>with</strong>out this restricti<strong>on</strong>.TABLE 7.14Factors for c<strong>on</strong>verting maximum micr<strong>on</strong>utrient amounts per 40 kcal-sizedservings to maximum amounts for different <strong>food</strong> presentati<strong>on</strong>s andserving sizesFood presentati<strong>on</strong> Usual serving size C<strong>on</strong>versi<strong>on</strong> factorPer usual serving sizePer 100 g or 100 mlSolid 50 g 4.0 8.0Milk or cereal-based 250 ml 5.0 2.0beveragesSugar-based beverages 250 ml 2.5 1.0These recommendati<strong>on</strong>s are in line <strong>with</strong> Codex guidelines <strong>on</strong> nutriti<strong>on</strong> claimsand their use, which are <strong>on</strong>ly expressed in terms of percentage of the NRVserving for minerals and vitamins (see secti<strong>on</strong> 7.5.3.1). The Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>for Use of Nutriti<strong>on</strong> Claims (343) stipulate that a <strong>food</strong> can <strong>on</strong>ly be describedas a “source” of a specific nutrient if it supplies 15% of the NRV per usualserving, (or 15% of the NRV per 100 g (solid <strong>food</strong>), or 5% of the NRV per100 ml (liquid <strong>food</strong>), or 5% of the NRV per 100 kcal). In order to qualifyas being “high” in a specific nutrient, a <strong>food</strong> product must c<strong>on</strong>tain twice asmuch of the nutrient as a “source” does. It means that many <strong>food</strong>s could beclassified as a “source”, but very few products – mostly those naturally rich inmicr<strong>on</strong>utrients – could be classified as “high” in specific micr<strong>on</strong>utrients.176
7. DEFINING AND SETTING PROGRAMME GOALIt is generally recommended that nutrient c<strong>on</strong>tent claims be restricted inaccordance <strong>with</strong> these rules, even if the <strong>food</strong> product c<strong>on</strong>tains – for technologicalpurposes or naturally – more than 30% of the NRV. Claims based <strong>on</strong> percentagesin excess of 30% of the NRV in a given fortified <strong>food</strong> should bediscouraged, <strong>on</strong> the grounds that such claims might mislead c<strong>on</strong>sumers as towhat c<strong>on</strong>stitutes a properly balanced diet.Summary■ Authorities taking the decisi<strong>on</strong> to launch a micr<strong>on</strong>utrient fortificati<strong>on</strong> programmeshould not do so <strong>with</strong>out collecting <strong>food</strong> and nutrient intake data, supported byvarious ancillary informati<strong>on</strong>, especially, biochemical data <strong>on</strong> nutriti<strong>on</strong>al status. Suchdata are necessary to make an informed judgment about the types and amountsof specific nutrients to add to which <strong>food</strong>s. Given the l<strong>on</strong>g-term effort and investmentthat is needed to implement and sustain fortificati<strong>on</strong> programmes, and theneed to protect individuals in populati<strong>on</strong>s c<strong>on</strong>suming the fortified <strong>food</strong>s, both fordeficiencies as well as for excesses, an initial investment in collecting adequate<strong>food</strong> intake data is highly recommended.— Biochemical and clinical data can reveal which micr<strong>on</strong>utrients are insufficient inthe usual diet and indicate the prevalence and severity of specific micr<strong>on</strong>utrientdeficiencies in different populati<strong>on</strong> groups.— Informati<strong>on</strong> <strong>on</strong> the distributi<strong>on</strong> of usual dietary intakes of nutrients <strong>with</strong>in populati<strong>on</strong>groups provides the most useful basis <strong>on</strong> which to justify and design amicr<strong>on</strong>utrient fortificati<strong>on</strong> programme to correct micr<strong>on</strong>utrient deficiencies.— Knowledge of dietary patterns, although useful, is not sufficient informati<strong>on</strong> formaking final decisi<strong>on</strong>s about which nutrients to add to which <strong>food</strong>s, and howmuch of each nutrient to add.■ The amount of micr<strong>on</strong>utrient added to the diet through <strong>food</strong> fortificati<strong>on</strong> should bedesigned such that the predicted probability of inadequate intakes of that specificnutrient is ≤2.5% for populati<strong>on</strong> subgroups of c<strong>on</strong>cern, while avoiding risk of excessiveintakes in other subgroups of the populati<strong>on</strong>.■ Due to technological, safety and cost c<strong>on</strong>straints, it may not be possible to add theamount of nutrient(s) needed to ensure adequate intakes in almost all members ofa populati<strong>on</strong> by mass fortificati<strong>on</strong>. In that case, fortificati<strong>on</strong> of several <strong>food</strong> vehicles,other types of fortificati<strong>on</strong>, or supplementati<strong>on</strong>, should be c<strong>on</strong>sidered.■ While these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> provide informati<strong>on</strong> <strong>on</strong> the rati<strong>on</strong>ale for fortificati<strong>on</strong> and theimplementati<strong>on</strong> of fortificati<strong>on</strong> programmes, the final decisi<strong>on</strong>s c<strong>on</strong>cerning whichmicr<strong>on</strong>utrients to prioritize in a specific locati<strong>on</strong> should be made <strong>on</strong> the basis oflocal informati<strong>on</strong> and public health priorities.177
CHAPTER 8M<strong>on</strong>itoring and evaluati<strong>on</strong>M<strong>on</strong>itoring and evaluati<strong>on</strong> are essential comp<strong>on</strong>ents of any <strong>food</strong> fortificati<strong>on</strong>programme, systems for which should be developed at the outset of a programme,ideally during the design and planning stages. M<strong>on</strong>itoring and evaluati<strong>on</strong>provide an opportunity not <strong>on</strong>ly to assess the quality of the implementati<strong>on</strong>and delivery of a programme, but also the degree to which it reaches its targetedhouseholds and individuals, and achieves its nutriti<strong>on</strong>al goals. More importantly,the results of m<strong>on</strong>itoring and evaluati<strong>on</strong> exercises provide programme plannersand policy-makers <strong>with</strong> the necessary informati<strong>on</strong> to make decisi<strong>on</strong>s aboutwhether to c<strong>on</strong>tinue, expand, replicate or end a programme.8.1 Basic c<strong>on</strong>cepts and definiti<strong>on</strong>sFor a fortificati<strong>on</strong> programme to be effective, the chosen <strong>food</strong> vehicles have tobe available nati<strong>on</strong>wide or, at least, in the specific geographical areas targeted bythe programme. In practice, this means that the product must be available topurchase from local retail stores or outlets that are accessible to the targeted segmentsof the populati<strong>on</strong>. Furthermore, the fortified products have to be purchasedby the target families, and c<strong>on</strong>sumed <strong>with</strong> sufficient frequency and inappropriate amounts by the targeted individuals. Throughout this process, thatis to say, from the factory to the retail stores, and right up until the time of c<strong>on</strong>sumpti<strong>on</strong>by targeted individuals, it is vital that the product maintains itsexpected quality. Thus to ensure that the planned impact is achieved, a programme’soperati<strong>on</strong>al performance (or implementati<strong>on</strong> efficiency) must be m<strong>on</strong>itored;this is best accomplished through a system of c<strong>on</strong>tinuous data collecti<strong>on</strong>at key delivery points. When bottlenecks or operati<strong>on</strong>al inefficiencies are identified,informati<strong>on</strong> must be directed to the programme entity resp<strong>on</strong>sible forimplementing remedial acti<strong>on</strong>s and for re-directing the programme as needed.This set of acti<strong>on</strong>s c<strong>on</strong>stitutes programme m<strong>on</strong>itoring.In the c<strong>on</strong>text of <strong>food</strong> fortificati<strong>on</strong>, the term “m<strong>on</strong>itoring” thus refers to thec<strong>on</strong>tinuous collecti<strong>on</strong>, review and use of informati<strong>on</strong> <strong>on</strong> programme implementati<strong>on</strong>activities, for the purpose of identifying problems, such as n<strong>on</strong>compliance,and informing corrective acti<strong>on</strong>s so as to fulfil stated objectives (6).The ultimate purpose of m<strong>on</strong>itoring a fortificati<strong>on</strong> programme is to ensure that178
8. MONITORING AND EVALUATIONthe fortified product, of the desired quality, is made available and is accessibleto c<strong>on</strong>sumers in sufficient amounts.The term “evaluati<strong>on</strong>” <strong>on</strong> the other hand is used to refer to the assessmentof the effectiveness and the impact of a programme <strong>on</strong> the target populati<strong>on</strong>. Inthe case of <strong>food</strong> fortificati<strong>on</strong>, evaluati<strong>on</strong>s are undertaken <strong>with</strong> the aim of providingevidence that the programme is indeed reaching its nutriti<strong>on</strong>al goals, bethis an increase in the intake of a fortified <strong>food</strong> or of specific nutrients, or animprovement in the nutriti<strong>on</strong>al status, health or functi<strong>on</strong>al outcomes of the targetpopulati<strong>on</strong>. Programme evaluati<strong>on</strong> should not be undertaken until a programmehas been shown – through appropriate m<strong>on</strong>itoring – that it has been implementedas planned, and is operating efficiently. A poorly implemented programmeis unlikely to achieve its desired impact, and thus, resources should notbe wasted in undertaking evaluati<strong>on</strong>s until programme operati<strong>on</strong>al inefficiencieshave been corrected.A schematic representati<strong>on</strong> of a model m<strong>on</strong>itoring and evaluati<strong>on</strong> system forfortificati<strong>on</strong> programmes is shown in Figure 8.1; this model provides a frameworkfor the various m<strong>on</strong>itoring and evaluati<strong>on</strong> activities that are described inthis chapter.The framework model distinguishes two main categories of m<strong>on</strong>itoring, regulatorym<strong>on</strong>itoring and household/individual m<strong>on</strong>itoring. The former, regulatorym<strong>on</strong>itoring, encompasses all m<strong>on</strong>itoring activities c<strong>on</strong>ducted at the producti<strong>on</strong>level (i.e. at factories, packers), as well as m<strong>on</strong>itoring at customs warehouses andFIGURE 8.1A m<strong>on</strong>itoring and evaluati<strong>on</strong> system for <strong>food</strong> fortificati<strong>on</strong> programmesFoodNati<strong>on</strong>al or importedVitamins premixCertificate of Quality(Food C<strong>on</strong>trol andCustoms)Importedfortified <strong>food</strong>Certificate ofC<strong>on</strong>formity orInspecti<strong>on</strong>(Corroboratingtrial) (FoodC<strong>on</strong>trol Dept. andCustoms)Quality auditing <strong>with</strong>C<strong>on</strong>formity Assessment(Food C<strong>on</strong>trol/witnesses)Internal m<strong>on</strong>itoring(factories or packers)Importati<strong>on</strong> warehouseExternal m<strong>on</strong>itoring(factories or packers)Commercial m<strong>on</strong>itoring(at retail stores)Household/individualm<strong>on</strong>itoringImpact evaluati<strong>on</strong>(individuals, households)Quality C<strong>on</strong>trol and QualityAssurance (Dept. of Quality C<strong>on</strong>trolof Factories and Packers)Factory Inspecti<strong>on</strong> (Corroboratingtrial) Technical Auditing (GovernmentFood C<strong>on</strong>trol Unit)Verificati<strong>on</strong> of Legal Compliance(Corroborating trial in retail stores)(Food C<strong>on</strong>trol and Units of Standardsand/or C<strong>on</strong>sumer Protecti<strong>on</strong>)Assessment of provisi<strong>on</strong>,utilizati<strong>on</strong> and coverageAssessment of impact <strong>on</strong>c<strong>on</strong>sumpti<strong>on</strong>, biochemicals,clinical and functi<strong>on</strong>al outcomeREGULATORYMONITORINGHOUSEHOLD/INDIVIDUALM<strong>on</strong>itoring andevaluati<strong>on</strong>179
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSat retail stores, by c<strong>on</strong>cerned regulatory authorities as well as by producers themselvesas part of self-regulati<strong>on</strong> programmes. Producti<strong>on</strong> level regulatory m<strong>on</strong>itoringcomprises both internal and external m<strong>on</strong>itoring; regulatory m<strong>on</strong>itoringat the retail level is referred to here as commercial m<strong>on</strong>itoring. The primary aimof regulatory m<strong>on</strong>itoring is to ensure that the fortified <strong>food</strong>s meet the nutrient,quality and safety standards set prior to programme implementati<strong>on</strong>.The other category, household/individual m<strong>on</strong>itoring, as its name implies,involves households and their members and has the following objectives(adapted from Habicht et al. (344):— to ensure that targeted individuals and households have access to the fortified<strong>food</strong> and that the fortified <strong>food</strong> is of the expected quality (i.e. tomeasure service provisi<strong>on</strong>);— to ensure that targeted individuals and households purchase and c<strong>on</strong>sumethe fortified <strong>food</strong> (i.e. to m<strong>on</strong>itor service utilizati<strong>on</strong>);— to ensure that targeted individuals and households c<strong>on</strong>sume the fortified<strong>food</strong> in appropriate amounts and frequency (i.e. to measure coverage).Once regulatory and household m<strong>on</strong>itoring have dem<strong>on</strong>strated that the programmeis operating in a satisfactory manner, evaluati<strong>on</strong> of the programme atthe household and at the individual level can be undertaken to assess its impact.This is generally referred to as impact evaluati<strong>on</strong> (Figure 8.1) Some of the dataobtained through household m<strong>on</strong>itoring, for example, data <strong>on</strong> c<strong>on</strong>sumpti<strong>on</strong> offortified <strong>food</strong>s and/or micr<strong>on</strong>utrient intakes, can also be used in programmeevaluati<strong>on</strong> (see secti<strong>on</strong> 8.4).Table 8.1 summarizes the key features of each of the three principal frameworkcomp<strong>on</strong>ents of m<strong>on</strong>itoring and evaluati<strong>on</strong> identified above, i.e. regulatorym<strong>on</strong>itoring, household m<strong>on</strong>itoring and impact evaluati<strong>on</strong>. The remainder of thischapter is devoted to discussing each of these comp<strong>on</strong>ents in more detail andc<strong>on</strong>cludes by outlining the minimum requirements for a m<strong>on</strong>itoring and evaluati<strong>on</strong>system for a fortificati<strong>on</strong> programme (secti<strong>on</strong> 8.5).8.2 Regulatory m<strong>on</strong>itoringAs shown in Figure 8.1, regulatory m<strong>on</strong>itoring comprises three parts – internalm<strong>on</strong>itoring, external m<strong>on</strong>itoring and commercial m<strong>on</strong>itoring:• Internal m<strong>on</strong>itoring refers to the quality c<strong>on</strong>trol and quality assurance(QC/QA) practices c<strong>on</strong>ducted by producers, importers and packers.• External m<strong>on</strong>itoring refers to the inspecti<strong>on</strong> and auditing activities carried outat producti<strong>on</strong> centres (factories and packers) and importati<strong>on</strong> custom sites.Governmental authorities are resp<strong>on</strong>sible for external m<strong>on</strong>itoring, which is180
8. MONITORING AND EVALUATIONTABLE 8.1Purpose and functi<strong>on</strong> of the various comp<strong>on</strong>ents of m<strong>on</strong>itoring and evaluati<strong>on</strong> systems for fortificati<strong>on</strong> programmesComp<strong>on</strong>ent Purpose Specific functi<strong>on</strong>Regulatory To ensure that fortified <strong>food</strong>s meet Regulatory m<strong>on</strong>itoring can address questi<strong>on</strong>s such as:m<strong>on</strong>itoring nutrient quality and safety standards — Is GMP applied?throughout their shelf-life (i.e. from — Is HACCP in place (when applicable)?factory to retail store); comprises: — Is QA/QC correctly d<strong>on</strong>e?— internal m<strong>on</strong>itoring; — Are inspecti<strong>on</strong> and technical auditing functi<strong>on</strong>s at the factory and at packing— external m<strong>on</strong>itoring; facilities implemented satisfactorily?— commercial m<strong>on</strong>itoring. — Is verificati<strong>on</strong> of legal compliance at retail stores carried out as planned?Household/ To assess: Household m<strong>on</strong>itoring can address questi<strong>on</strong>s such as:individual — provisi<strong>on</strong>; — Is the fortified <strong>food</strong> accessible to the target populati<strong>on</strong>?m<strong>on</strong>itoring — utilizati<strong>on</strong>; — Is the fortified <strong>food</strong> of acceptable quality?and — coverage. — Does the targeted populati<strong>on</strong> purchase the fortified <strong>food</strong>?evaluati<strong>on</strong> — Is the fortified <strong>food</strong> being stored, handled/prepares as intended?— Does the targeted populati<strong>on</strong> c<strong>on</strong>sume the fortified <strong>food</strong> in appropriateamounts/frequency?Impact To assess impact <strong>on</strong> outcomes of Impact evaluati<strong>on</strong>s can address questi<strong>on</strong>s such as:evaluati<strong>on</strong> interest, such as: — Has the targeted populati<strong>on</strong> reached a pre-established acceptable level of a— c<strong>on</strong>sumpti<strong>on</strong> of fortified <strong>food</strong>; given outcome of interest (e.g. is prevalence of ir<strong>on</strong> deficiency
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSimplemented as a mechanism to assure compliance <strong>with</strong> standards andregulati<strong>on</strong>s.• Commercial m<strong>on</strong>itoring is similar to external m<strong>on</strong>itoring in that it is generallythe resp<strong>on</strong>sibility of the government and its purpose is to verify that the fortifiedproducts comply <strong>with</strong> standards, but is c<strong>on</strong>ducted at the level of retailstores.For each stage of the m<strong>on</strong>itoring process, it is helpful to establish indicators thatcan be used to measure success. In the case of fortificati<strong>on</strong> programmes, successcriteria can be expressed in terms of the proporti<strong>on</strong> of samples c<strong>on</strong>taining aspecified minimum amount of a given nutrient at various stages in the lifecycleof the product, i.e. at the time of producti<strong>on</strong> (the Producti<strong>on</strong> Minimum), at thepoint of sale (the Retail or Legal Minimum) and at the point of c<strong>on</strong>sumpti<strong>on</strong>(the Household Minimum). A sample set of success criteria for m<strong>on</strong>itoring purposesare presented in Table 8.2.To be effective, a m<strong>on</strong>itoring system requires a set of established procedures,methodologies and reporting requirements, all of which make a c<strong>on</strong>tributi<strong>on</strong>to ensuring the c<strong>on</strong>tinuous assessment of a programme. A clear delineati<strong>on</strong> ofresp<strong>on</strong>sibilities and an efficient feedback mechanism, which facilitate the establishmentand implementati<strong>on</strong> of corrective measures when operati<strong>on</strong>al problemsarise, are also essential. Table 8.3 (345) outlines how some of these facets ofTABLE 8.2Suggested criteria for measuring success at various m<strong>on</strong>itoring stages for<strong>food</strong> fortificati<strong>on</strong> programmes (expressed as a percentage of samples thatmust comply <strong>with</strong> minimum levels and Maximum Tolerable Levels)M<strong>on</strong>itoring Minimum levels MaximumstageTolerableHousehold a Retail b Producti<strong>on</strong> c Level dInternal 100 100 ≥80
8. MONITORING AND EVALUATIONTABLE 8.3Suggested regulatory m<strong>on</strong>itoring activities for a <strong>food</strong> fortificati<strong>on</strong> programmeM<strong>on</strong>itoring Acti<strong>on</strong>/indicator (success Frequency/timing Methodology and entity resp<strong>on</strong>sible for acti<strong>on</strong>stage criteria)Internal GMP applied Daily. Method: Follow a GMP manual approved bym<strong>on</strong>itoring company directors.(quality HACCP system in place, where Daily. Resp<strong>on</strong>sible: Factory manager.c<strong>on</strong>trol and applicable Method: Follow a HACCP manual approved byassurance) company directors.Resp<strong>on</strong>sible: Factory manager.Premixes and preblends available Daily. Method: C<strong>on</strong>tinuous inventory of micr<strong>on</strong>utrientin sufficient amounts for at least premixes and preblends in existence and use.15 days of producti<strong>on</strong> C<strong>on</strong>firm that batches of premix are used in thesame order in which they were produced.Resp<strong>on</strong>sible: Factory manager.Dosage is in the correct At least <strong>on</strong>ce per shift. Method: Ensure premix flows according to theproporti<strong>on</strong> producti<strong>on</strong> rate so that the theoretical average isas expected and the Producti<strong>on</strong> Minimum Levelis always attained.Resp<strong>on</strong>sible: Factory quality c<strong>on</strong>trol department.Corroborating tests (at least 80% At least every 8 hours; if success Method: Take a random sample(s) from packagingof samples fulfil the Producti<strong>on</strong> criteria are not fulfilled, line. A fast semi-quantitative assay can be used atMinimum Level and less than frequency of sampling should be shorter intervals, but at least <strong>on</strong>e daily-composite20% reach the Maximum increased to every 2–4 hours. sample should be analysed using a quantitativeTolerable Level) assay.Resp<strong>on</strong>sible: Factory quality c<strong>on</strong>trol department.183
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 8.3Suggested regulatory m<strong>on</strong>itoring activities for a <strong>food</strong> fortificati<strong>on</strong> programme (C<strong>on</strong>tinued)M<strong>on</strong>itoring Acti<strong>on</strong>/indicator (success Frequency/timing Methodology and entity resp<strong>on</strong>sible for acti<strong>on</strong>stage criteria)ExternalFactory Fortificati<strong>on</strong> centre carries out At least <strong>on</strong>ce every 3–6 m<strong>on</strong>ths; Method: C<strong>on</strong>duct auditing to verify performance of(inspecti<strong>on</strong> QC/QA procedures and maintains frequency of visits should be the QC/QA procedures and registry, and thatand up-to-date registers increased to 1–4 times/m<strong>on</strong>th if fortificati<strong>on</strong> centres adopt GMP.technical problems are detected. Resp<strong>on</strong>sible: Food c<strong>on</strong>trol authorities.auditing) Corroborating tests (at least 80% Combine testing <strong>with</strong> visits to Method: Collect 5 individual samples of packagedof individual samples fulfil the examine QC/QA and GMP product and take 5 samples from the producti<strong>on</strong>Legal Minimum Level and less procedures; if intenti<strong>on</strong>al or line, and test for compliance.than 20% reach the Maximum serious mistakes are suspected, Resp<strong>on</strong>sible: Food c<strong>on</strong>trol authorities.Tolerable Level) plan a Quality Audit forEvaluati<strong>on</strong> of C<strong>on</strong>formity.At importati<strong>on</strong> Obtain Certificate of C<strong>on</strong>formity a Each time a product lot enters Method: Examine documentati<strong>on</strong>, quality andsites (applies of sale from country of origin the country. labelling of products in the customs warehouses.to imported/ Resp<strong>on</strong>sible: Importati<strong>on</strong> officials in collaborati<strong>on</strong> <strong>with</strong>d<strong>on</strong>ated <strong>food</strong> c<strong>on</strong>trol authorities.products) Corroborating tests (at least 80% Combine <strong>with</strong> examinati<strong>on</strong> of Method: Randomly select 5 individual samples fromof individual samples fulfill the importati<strong>on</strong> papers. If intenti<strong>on</strong>al the lot and test for compliance <strong>with</strong> the LegalLegal Minimum Level and less or serious mistakes are Minimum Level and the MTL.than 20% reach the Maximum suspected, plan a Quality Audit Resp<strong>on</strong>sible: Importati<strong>on</strong> officials in collaborati<strong>on</strong> <strong>with</strong>Tolerable Level) for Evaluati<strong>on</strong> of C<strong>on</strong>formity. <strong>food</strong> c<strong>on</strong>trol authorities.184
8. MONITORING AND EVALUATIONCommercial Corroborating tests (at least 80% Systematic and c<strong>on</strong>tinuous Method: Visit stores to collect samples; send samples(inspecti<strong>on</strong> at of samples of each brand fulfill examinati<strong>on</strong> of the product to official laboratories for quantitative assays. At theretail stores) the Legal Minimum Level and distributed to all regi<strong>on</strong>s of the local level, semi-quantitative assays may also beless than 20% reach the country; each regi<strong>on</strong> should be used to c<strong>on</strong>firm presence of fortificant if fraud isMaximum Tolerable Level) visited at least <strong>on</strong>ce a year. suspected.Resp<strong>on</strong>sible: Local pers<strong>on</strong>nel from public instituti<strong>on</strong>s(e.g. representatives of ministries of health,industry, c<strong>on</strong>sumer protecti<strong>on</strong> organizati<strong>on</strong>s).Quality Audit Verify producti<strong>on</strong> or stored batch Whenever it is necessary to take Method: Visit fortificati<strong>on</strong> centres suspected of n<strong>on</strong>forEvaluati<strong>on</strong> complies <strong>with</strong> standards when legal acti<strong>on</strong>s; can also be compliance <strong>with</strong> regulati<strong>on</strong>s and standards, orof C<strong>on</strong>formity analysed using statistical requested and financed by when required by exporting industry. Followsampling criteria producers to certify producti<strong>on</strong> technical recommendati<strong>on</strong>s of the Codexlot for exportati<strong>on</strong>. Alimentarius Commissi<strong>on</strong> (345) or any equivalentguidelines suitable for this activity.Resp<strong>on</strong>sible: Pers<strong>on</strong>nel of the public agency for <strong>food</strong>c<strong>on</strong>trol: as visits to fortificati<strong>on</strong> centres areperformed under suspici<strong>on</strong>s of n<strong>on</strong>-compliance ofregulati<strong>on</strong>s and standards, these activities shouldbe carried out in the presence of independentwitnesses.GMP, good manufacturing practice; HACCP, hazard analysis and critical c<strong>on</strong>trol point; MTL, Maximum Tolerable Level; QC/QA, quality c<strong>on</strong>trol/qualityassurance.aThe Certificate of C<strong>on</strong>formity is a statement that the imported product complies <strong>with</strong> a set of specific standards.185
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSm<strong>on</strong>itoring might be implemented in practice for each of the three stages of regulatorym<strong>on</strong>itoring, internal, external and commercial.Table 8.3 lists an additi<strong>on</strong>al m<strong>on</strong>itoring stage, namely quality audits for evaluati<strong>on</strong>of c<strong>on</strong>formity. This is the formal examinati<strong>on</strong> and testing of a batch of afortified <strong>food</strong> product for compliance <strong>with</strong> standards. It should be reserved forspecial circumstances, which can either be when intenti<strong>on</strong>al n<strong>on</strong>-c<strong>on</strong>formity issuspected (and legal acti<strong>on</strong> is required) or when certificati<strong>on</strong> of a producti<strong>on</strong> lotprior to exportati<strong>on</strong> is necessary.8.2.1 Internal m<strong>on</strong>itoring (quality c<strong>on</strong>trol/quality assurance)Broadly speaking, quality assurance (QA) refers to the implementati<strong>on</strong> ofplanned and systematic activities necessary to ensure that products or servicesmeet quality standards. The performance of quality assurance can be expressednumerically in terms of the results of quality c<strong>on</strong>trol procedures. Quality c<strong>on</strong>trol(QC) is defined as the techniques and assessments used to document compliance<strong>with</strong> established technical standards, through the use of objective and measurableindicators that are applicable to the products or services.Detailed informati<strong>on</strong> about QC/QA can be found in any <strong>on</strong>e of the manytechnical manuals that are devoted to this subject and in publicati<strong>on</strong>s <strong>on</strong> goodmanufacturing practice (GMP) (346). In these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> the topic of QA/QCis viewed from a purely public health perspective and focuses <strong>on</strong> indicators andcriteria that are relevant to the process of <strong>food</strong> fortificati<strong>on</strong>. Thus in the c<strong>on</strong>textof <strong>food</strong> fortificati<strong>on</strong>, quality assurance c<strong>on</strong>sists of establishing the followingprocedures:— obtain from the providers a certificate of quality 1 for any micr<strong>on</strong>utrientmixes used;— request, receive and store in a systematic, programmed and timely mannerthe ingredients and supplies for the preparati<strong>on</strong> of a preblend 2 ;— produce the preblend according to a schedule that is adjusted to the rateof <strong>food</strong> manufacturing and fortificati<strong>on</strong>;— c<strong>on</strong>trol the adequate performance of the preblend equipment;— appropriately label and deliver the preblend;1The micr<strong>on</strong>utrient mixes must be accompanied by a document that certifies their nutrient c<strong>on</strong>tent.This is usually the case for products shipped by internati<strong>on</strong>al companies dedicated to this task.2A preblend is the combinati<strong>on</strong> of the micr<strong>on</strong>utrient mix <strong>with</strong> another ingredient, often the same<strong>food</strong> that is to be fortified, <strong>with</strong> the purpose of reducing the diluti<strong>on</strong> proporti<strong>on</strong> and improvingthe distributi<strong>on</strong> of the micr<strong>on</strong>utrient mix in the <strong>food</strong> and guaranteeing that there will be not beseparati<strong>on</strong> (segregati<strong>on</strong>) between the <strong>food</strong> and micr<strong>on</strong>utrient particles.186
8. MONITORING AND EVALUATION— use the preblend in the same order of producti<strong>on</strong> (i.e. first in, first out);—verify appropriate functi<strong>on</strong>ing of the feeder machines and the mixers in ac<strong>on</strong>tinuous and systematic manner;— ensure that the product is adequately packaged, labelled, stored andshipped.It is possible that other process variables, such as pH and temperature/timeexposure, could affect the stability of added micr<strong>on</strong>utrients and should also bec<strong>on</strong>sidered in the design of quality assurance programmes.The quality c<strong>on</strong>trol procedures will typically c<strong>on</strong>sist of taking samples of thefortified <strong>food</strong>, either by batch or in a c<strong>on</strong>tinuous manner depending <strong>on</strong> thesystem of producti<strong>on</strong>, and determining their micr<strong>on</strong>utrient c<strong>on</strong>tent. Irrespectiveof the sampling method, the number of samples required will be governed bythe c<strong>on</strong>sistency and reliability of the fortificati<strong>on</strong> process. A highly homogeneousand c<strong>on</strong>sistent operati<strong>on</strong>, regardless of the size of the batch or the rate of producti<strong>on</strong>,will need less sampling than <strong>on</strong>e <strong>with</strong> variable results. Nevertheless,even in the most reproducible c<strong>on</strong>diti<strong>on</strong>s it is important to take and analysesamples routinely in order to verify and keep track of whether the technical standardsare being met.Figure 8.2 illustrates the features of a dynamic sampling system suitable fora c<strong>on</strong>tinuous producti<strong>on</strong> process. Under optimal operati<strong>on</strong>, <strong>on</strong>e sample of aproduct per 8-hour shift might be sufficient; this would be categorized as arelaxed intensity of sampling. If the technical specificati<strong>on</strong>s of the product arenot attained (i.e. the micr<strong>on</strong>utrient c<strong>on</strong>tent is lower than the factory minimumor higher than the Maximum Tolerable Level), then sampling frequency shouldFIGURE 8.2Suggested frequency and intensity of sampling for m<strong>on</strong>itoring compliance<strong>with</strong> standardsWarning Warning AUDITINGFailures1 2/5 2/5C<strong>on</strong>sumer’srisk{RELAXEDNORMALDEMANDING{Producer’sriskSuccessesIDEAL 3 3Sampling frequency• Internal m<strong>on</strong>itoring 8 hours 4 hours 2 hours• External m<strong>on</strong>itoring 3 m<strong>on</strong>ths M<strong>on</strong>thly 15 days187
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSbe increased from the relaxed intensity to a normal intensity and correctiveacti<strong>on</strong>s taken. In a “normal” situati<strong>on</strong>, if 2 out of 5 c<strong>on</strong>secutive samples of theproduct fail to meet the technical requirements, then the intensity of samplingshould be changed to a demanding intensity, and corrective acti<strong>on</strong>s implemented.Again, if 2 out of 5 c<strong>on</strong>secutive samples do not achieve the technical requirements,then the producti<strong>on</strong> should be stopped until the source of error is foundand the necessary corrective measures introduced.When producti<strong>on</strong> is reinitiated, sampling should start at the demanding intensity,be switched to a normal and then back down to a relaxed intensity if, eachtime, three c<strong>on</strong>secutive samples satisfy technical requirements. The relaxed situati<strong>on</strong>implies a degree of c<strong>on</strong>sumer risk; if sampling is infrequent, there is agreater chance of some n<strong>on</strong>-compliant batches reaching the market. When samplingis frequent (i.e. as in the demanding situati<strong>on</strong>), the likelihood of detectingeven a minor deviati<strong>on</strong> from the standard is increased, and prompts producersto expend time and resources in the resoluti<strong>on</strong> of the problem (the producer’srisk). Neither the relaxed nor the normal sampling intensity should be viewedas positive or negative; they simply reflect the performance of the fortificati<strong>on</strong>process at the moment of the assessment. Results of QC must be carefullyrecorded and kept, because they document the history of the efficiency and alsothe producer’s supervisi<strong>on</strong> of the fortificati<strong>on</strong> process.Because results are needed quickly (so that corrective acti<strong>on</strong>s can be implementedpromptly), QC procedures demand fast and simple analytical assays.These assays do not necessarily need to have high analytical resoluti<strong>on</strong> (i.e. beable to discriminate between small c<strong>on</strong>centrati<strong>on</strong> ranges), but it is essential thatthey are able to determine whether fortificati<strong>on</strong> standards are being met (i.e.micr<strong>on</strong>utrient c<strong>on</strong>tent not less than the producti<strong>on</strong> minimum nor more than theMaximum Tolerable Level). In this regard, semi-quantitative assays are potentiallyvery useful and attempts have been made in recent years to develop testkits based <strong>on</strong> semi-quantitative assays. Test kits for the measurement of iodinein salt, for example, have been developed but, to date, have met <strong>with</strong> limitedsuccess; those currently <strong>on</strong> the market, have been found to be of questi<strong>on</strong>ablereliability (347). Clearly, further research is needed in this area before semiquantitativetests can be applied more widely in the <strong>food</strong> industry.8.2.2 External m<strong>on</strong>itoring (inspecti<strong>on</strong> and technical auditing)Some form of external m<strong>on</strong>itoring by governmental <strong>food</strong> c<strong>on</strong>trol authorities isessential to assure that producers are complying <strong>with</strong> the approved technicalstandards that ensure the quality and safety of <strong>food</strong> fortificati<strong>on</strong>. Awareness thattheir product might be checked at any time usually provides producers <strong>with</strong> astr<strong>on</strong>g motivati<strong>on</strong>al force to carry out an acceptable producti<strong>on</strong> process <strong>with</strong>the appropriate QA/QC procedures. In industrialized countries, it is usually188
8. MONITORING AND EVALUATIONsufficient to c<strong>on</strong>firm <strong>on</strong>ce a year (or even less frequently) the nutrient c<strong>on</strong>tentindicated <strong>on</strong> the <strong>food</strong> labels from samples taken in the market (see Commercialm<strong>on</strong>itoring, secti<strong>on</strong> 8.2.3). However, in much of the developing world, where itis very difficult to trace and to retrieve a defective batch <strong>on</strong>ce a <strong>food</strong> productreaches retail stores, it is advisable to also c<strong>on</strong>duct external m<strong>on</strong>itoring at thefactory level.External m<strong>on</strong>itoring combines two types of acti<strong>on</strong>s:• checking the performance and the records of the producers’ quality assuranceprocedures (technical auditing);• c<strong>on</strong>firming that the technical specificati<strong>on</strong>s for the product are being met atfactories, packaging sites and points of entry into the country (inspecti<strong>on</strong>).Ideally, inspecti<strong>on</strong> and verificati<strong>on</strong> of legal compliance should be based <strong>on</strong> theanalytical assessment of the micr<strong>on</strong>utrient c<strong>on</strong>tent of a <strong>food</strong> product by meansof a quantitative assay. All samples should c<strong>on</strong>tain the fortificant; at least 80%of samples from factories, importati<strong>on</strong> sites and warehouses should present theLegal Minimum amount, and less than 20% of samples should have a micr<strong>on</strong>utrientc<strong>on</strong>tent that is above but always near the Maximum Tolerable Level (Table8.2). If this is found not to be the case, then more frequent visits to the factoryto carry out technical auditing and inspecti<strong>on</strong> activities are justified (seeFigure 8.2).Imported products should be treated in a similar manner to locally produced<strong>food</strong>s, <strong>on</strong>ly instead of checking the producers’ documented AQ/QC procedures,the certificate of c<strong>on</strong>formity provided by the country of origin should be examined.However, <strong>food</strong> c<strong>on</strong>trol authorities can corroborate compliance of the technicalstandards in samples of the imported shipments.The intensity of sampling and factory inspecti<strong>on</strong> frequency depends <strong>on</strong> thereproducibility of the fortificati<strong>on</strong> process and should be determined for eachtype of industry under the specific c<strong>on</strong>diti<strong>on</strong>s of each country. For example, forsalt fortificati<strong>on</strong> by small industries this might be every 15 days, for sugar industriesevery m<strong>on</strong>th, and for wheat flour industries every 6 m<strong>on</strong>ths. In theory, samplingshould follow a statistically based approach, such as that recommended bythe Codex Alimentarius Commissi<strong>on</strong> (345). In practice, however, the numberof samples and the analytical work required can overwhelm the available human,financial and material resources of the <strong>food</strong> c<strong>on</strong>trol entities in many developingcountries. For day-to-day m<strong>on</strong>itoring and routine inspecti<strong>on</strong> visits, the Codexsampling procedures are often impractical and unrealistic and are thus bestreserved for situati<strong>on</strong>s that require a quality audit for evaluati<strong>on</strong> of c<strong>on</strong>formity(e.g. for cases when the product requires a certificate of c<strong>on</strong>formity for exportati<strong>on</strong>,or if there is a legal c<strong>on</strong>troversy that might lead to serious penalties)(See Table 8.3).189
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSA simpler, low-cost m<strong>on</strong>itoring system, based <strong>on</strong> the c<strong>on</strong>cept of corroboratingtests, has been successfully adopted by some countries in Central America.Thesetests c<strong>on</strong>sist of checking compliance <strong>with</strong> fortificati<strong>on</strong> standards in a smallnumber of samples (e.g. 5–10 product samples from factories) during themoment of the technical auditing visit; samples are taken from the producti<strong>on</strong>line and also from storage areas. At least 80% of the samples should c<strong>on</strong>tain theLegal Minimum of the micr<strong>on</strong>utrient, and less than 20% should be above, butnever too far from the Maximum Tolerable Level. If these criteria are not fulfilled,then a warning statement must be provided and more frequent visits fortechnical auditing and inspecti<strong>on</strong> should be planned to the factories resp<strong>on</strong>siblefor the product. In extreme cases, a quality audit for evaluati<strong>on</strong> of c<strong>on</strong>formitymight be necessary (Table 8.3). The c<strong>on</strong>cept of corroborating tests is based <strong>on</strong>the principle that quality is the main resp<strong>on</strong>sibility of producers; governmentalauthorities <strong>on</strong>ly act to represent the public, and to guarantee that m<strong>on</strong>itoring isindeed carried out.8.2.3 Commercial m<strong>on</strong>itoringAs is the case for any other industrially produced <strong>food</strong>, fortified <strong>food</strong>s, irrespectiveof whether fortificati<strong>on</strong> is voluntary or in resp<strong>on</strong>se to public health interest,must be correctly identified <strong>with</strong> a label. A label should include at least thebrand of the product, the address of the resp<strong>on</strong>sible entity, and the LegalMinimum Level of the nutrient and, if industry development allows, also thedate of minimum durability, the batch number and the producti<strong>on</strong> date.As menti<strong>on</strong>ed in the previous secti<strong>on</strong>, in industrialized countries external regulatorym<strong>on</strong>itoring is generally limited to a c<strong>on</strong>firmati<strong>on</strong> of micr<strong>on</strong>utrientc<strong>on</strong>tent and label claims in samples obtained from retail stores. In the event ofa breach of standards, mechanisms exist for the recall of defective products andretracti<strong>on</strong> of misleading claims. Strict governmental enforcement of regulati<strong>on</strong>sand stiff penalties for n<strong>on</strong>-compliance, means that it is very rare for a producerto take the risk of not complying <strong>with</strong> regulati<strong>on</strong>s and <strong>on</strong> the whole, the procedureworks well. In the developing world, as indicated previously, it is not alwayspossible to trace and retrieve a defective batch <strong>on</strong>ce a <strong>food</strong> product reaches themarket place, and so it is necessary to m<strong>on</strong>itor for compliance <strong>with</strong> qualitystandards and label claims at the both the factory and retail levels (see alsosecti<strong>on</strong> 8.2.2).In many settings, especially in the developing world, commercial m<strong>on</strong>itoringcan be particularly useful for identifying brands and factories that deserve closerauditing. A system based <strong>on</strong> the use of corroborating tests, as suggested for factoriesabove, can equally well be applied to the commercial setting; <strong>on</strong>e or twosamples of each brand from each store might be used to check for standard compliance.If anomalies are found, then a technical comprehensive auditing of the190
8. MONITORING AND EVALUATIONresp<strong>on</strong>sible factory or importati<strong>on</strong> firm would be warranted. Semi-quantitativeassays to detect the presence of micr<strong>on</strong>utrients might be useful for m<strong>on</strong>itoringat retail stores, and as an enforcement tool at the local level. However, any legalacti<strong>on</strong> must be based <strong>on</strong> results obtained from quantitative assays carried out aspart of a quality auditing visit to the resp<strong>on</strong>sible factory.A nati<strong>on</strong>-wide programme to fortify vegetable oils <strong>with</strong> vitamins A and D 3was established in Morocco in 2002. The system devised for m<strong>on</strong>itoring thequality of the fortified product is described in detail in Annex E, and serves toillustrate the practical applicati<strong>on</strong> of the principles of regulatory m<strong>on</strong>itoringintroduced here.8.3 Household m<strong>on</strong>itoringIt is generally assumed that having established through regulatory m<strong>on</strong>itoringthat a fortified product is of the required quality at the retail store level, thesame product will be of a similar quality <strong>on</strong>ce it reaches households andindividuals. Because the product may have deteriorated during storage, c<strong>on</strong>firmati<strong>on</strong>of this assumpti<strong>on</strong> is always recommended. Nor can it be assumed thatjust because fortified <strong>food</strong>s are available to buy from shops, they will necessarilybe c<strong>on</strong>sumed by the target populati<strong>on</strong>; c<strong>on</strong>sumers may well purchase n<strong>on</strong>fortified<strong>food</strong>s in preference to fortified products (if both fortified andn<strong>on</strong>-fortified <strong>food</strong>s are available locally). Even if officially <strong>on</strong>ly fortified productsare available at retail stores, c<strong>on</strong>sumers may be able to acquire n<strong>on</strong>fortified(probably cheaper) <strong>food</strong>s via n<strong>on</strong>-official means, such as smugglingand from door-to-door sellers.8.3.1 Aims and objectivesIn short, the aim of household m<strong>on</strong>itoring is to assess whether or not a programmeis providing appropriately fortified products in sufficient amounts andat affordable prices to the targeted populati<strong>on</strong>. More specifically, householdm<strong>on</strong>itoring can answer questi<strong>on</strong>s such as:• Are the fortified products accessible (i.e. available and affordable) to the targetedhouseholds and individuals? Are they of expected quality and are theyavailable from retail stores in targeted regi<strong>on</strong>s/communities?• Are the fortified products being purchased by the targeted households, takinginto account tastes and preferences, and patterns of c<strong>on</strong>sumpti<strong>on</strong>? If not, whyare the fortified products not being purchased? Is it because they are unaffordable(cost), because their taste and appearance is altered by the fortificati<strong>on</strong>process, or is it because they are not part of the usual c<strong>on</strong>sumpti<strong>on</strong>pattern of the targeted populati<strong>on</strong>?191
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS• Are the fortified products being purchased and c<strong>on</strong>sumed in sufficientamounts by specific household members to meet programme nutriti<strong>on</strong>al goals(i.e. to increase their micr<strong>on</strong>utrient intake and/or to meet a predefined levelof micr<strong>on</strong>utrient requirements)? If not, is it because of cultural practices c<strong>on</strong>cerningthe appropriateness of feeding these products to specific householdmembers (based <strong>on</strong> age, physiological status, etc.), or is it because of tastesand preferences of specific household members, or because of inequitable<strong>food</strong> distributi<strong>on</strong> <strong>with</strong>in the household?• Which individuals/populati<strong>on</strong> groups are not being reached by the fortificati<strong>on</strong>programme and why?• Are individual family members c<strong>on</strong>suming sufficient amounts of fortifiedproducts to increase their intake of specific micr<strong>on</strong>utrients (and/or to meetprogramme nutriti<strong>on</strong>al goals for specific age/physiological groups)?In effect, m<strong>on</strong>itoring at the household level addresses three key aspectsof programme performance, that is to say, provisi<strong>on</strong>, utilizati<strong>on</strong> and coverage(see secti<strong>on</strong> 8.1). Household m<strong>on</strong>itoring activities designed to assess eachof these aspects of <strong>food</strong> fortificati<strong>on</strong> programme performance are outlined inTable 8.4; in each case, suitable indicators and data collecti<strong>on</strong> methodologiesare proposed.8.3.2 Methodological c<strong>on</strong>siderati<strong>on</strong>sAs Table 8.4 indicates, there are a variety of approaches that can be used togather data for the purposes of assessing programme performance in terms ofprovisi<strong>on</strong>, utilizati<strong>on</strong> and coverage. Primary data collecti<strong>on</strong> as part of the programme’soverall m<strong>on</strong>itoring and evaluati<strong>on</strong> system is <strong>on</strong>e opti<strong>on</strong>. Alternatively,and this is often a more practical soluti<strong>on</strong>, it may be possible to join <strong>with</strong> – or“piggy-back” <strong>on</strong> to – other programmes that have <strong>on</strong>going or regular data collecti<strong>on</strong>comp<strong>on</strong>ents. For example, some countries in Central America carry outschool censuses at regular intervals; it is then a relatively simple matter to collectsamples of fortified products, such as vitamin A-fortified sugar or iodized salt,by asking pupils to bring a small sample from home to school. Other routinedata collecti<strong>on</strong> systems which might provide opportunities for “piggy-backing”include 30-cluster surveys, sentinel sites m<strong>on</strong>itoring, and lot quality assurancesampling (LQAS) m<strong>on</strong>itoring systems (6,348–350).These types of simple m<strong>on</strong>itoringsystems are widely used to m<strong>on</strong>itor immunizati<strong>on</strong> coverage, universal saltiodizati<strong>on</strong> and other primary health care interventi<strong>on</strong>s. If such systems are notalready in place, they can be established specifically for the fortificati<strong>on</strong> programme.Guidance <strong>on</strong> how to implement relatively simple data collecti<strong>on</strong>systems, and examples of their successful applicati<strong>on</strong> in health care settings, isavailable elsewhere (6,351–353).192
8. MONITORING AND EVALUATIONTABLE 8.4Suggested household m<strong>on</strong>itoring activities for a <strong>food</strong> fortificati<strong>on</strong> programmeAspect of Indicator (success Frequency/timing Methodology and entity resp<strong>on</strong>sible for acti<strong>on</strong>programme criteria)performanceProvisi<strong>on</strong> Volume of product sold at At least annually. Method: Either through new data collecti<strong>on</strong> or by adding appropriate questi<strong>on</strong>san affordable price in (i.e. “piggy-backing”) <strong>on</strong>to existing data collecti<strong>on</strong> vehicles, such as:retail stores in target — cross-secti<strong>on</strong>al community surveys;regi<strong>on</strong>s (specific criteria — cross-secti<strong>on</strong>al household surveys;to be determined) — school surveys or censuses;— 30-cluster surveys;— sentinel site m<strong>on</strong>itoring;— lot quality assurance sampling (LQAS);— market surveys.Resp<strong>on</strong>sible: Programme m<strong>on</strong>itoring and evaluati<strong>on</strong> unit (if applicable), individualsresp<strong>on</strong>sible for the existing data collecti<strong>on</strong> programme that is being added to, orresearchers.Utilizati<strong>on</strong> Number or proporti<strong>on</strong> of At least annually. Method: As for Provisi<strong>on</strong>, excluding market surveys which do not apply here.households purchasing Resp<strong>on</strong>sible: As for Provisi<strong>on</strong>.fortified productregularlyNumber or proporti<strong>on</strong> oftargeted households inwhich fortified productis presentNumber or proporti<strong>on</strong> ofhousehold membersc<strong>on</strong>suming fortifiedproduct regularly193
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 8.4Suggested household m<strong>on</strong>itoring activities for a <strong>food</strong> fortificati<strong>on</strong> programme (C<strong>on</strong>tinued)Aspect of Indicator (success Frequency/timing Methodology and entity resp<strong>on</strong>sible for acti<strong>on</strong>programme criteria)performanceCoverage Proporti<strong>on</strong> of households Once a year until Method: Household surveys, either specific to the programme or as an add-<strong>on</strong> toor household members acceptable existing or planned surveys, depending <strong>on</strong> availability of resources locally. Inc<strong>on</strong>suming product <strong>with</strong> coverage to derive an appropriate denominator for coverage estimates, a representativeexpected frequency levels are order sample of the target populati<strong>on</strong> is, however, required.and in adequate achieved; Resp<strong>on</strong>sible: Programme m<strong>on</strong>itoring and evaluati<strong>on</strong> unit (if applicable) oramounts to meet thereafter every researchers.programme nutriti<strong>on</strong>al 3–5 years.goals (acceptable levelto be determined)Observed changes innutriti<strong>on</strong>al status sinceimplementati<strong>on</strong> offortificati<strong>on</strong> programmethrough intake offortified products andregular diet (acceptablechanges to bedetermined)194
8. MONITORING AND EVALUATIONMarket surveys are <strong>on</strong>e way of collecting data <strong>on</strong> the price and availability offortified products in retail stores; such data are useful for m<strong>on</strong>itoring serviceprovisi<strong>on</strong>. Many countries already operate routine systems for collecting pricedata for a number of <strong>food</strong> commodities, in which case fortified <strong>food</strong>s can simplybe added to the list of products being m<strong>on</strong>itored. The m<strong>on</strong>itoring of programmeutilizati<strong>on</strong> and coverage, however, necessitates data collecti<strong>on</strong> at the householdor individual level. Any of the simple data collecti<strong>on</strong> systems menti<strong>on</strong>ed abovecan be used to collect informati<strong>on</strong> relating to utilizati<strong>on</strong>. C<strong>on</strong>ducting representativehousehold and community surveys is another opti<strong>on</strong>, but these tend to bemore costly. Again, it is possible to take advantage of, or piggy-back <strong>on</strong>, existingdata collecti<strong>on</strong> vehicles or surveys that are being c<strong>on</strong>ducted at the householdlevel. In additi<strong>on</strong>, qualitative approaches, which include observati<strong>on</strong>s,informant interviews and focus group discussi<strong>on</strong>s, may be useful for gatheringinformati<strong>on</strong> about programme implementati<strong>on</strong> and service delivery, use of thefortified products and users’ percepti<strong>on</strong>s about the fortified versus the n<strong>on</strong>fortified<strong>food</strong>s.Coverage of a fortificati<strong>on</strong> programme is usually assessed by determiningwhat proporti<strong>on</strong> of at-risk individuals c<strong>on</strong>sume the fortified products in sufficientamounts and <strong>with</strong> sufficient frequency. Thus, to evaluate coverage, informati<strong>on</strong><strong>on</strong> the number of at-risk individuals is necessary. This can be obtainedfrom either a census or by surveying a representative sample of the populati<strong>on</strong>.Estimates of the intake of the fortified product(s) and/or of the micr<strong>on</strong>utrient(s)of interest are also required.Two approaches are available for evaluating programme coverage. The firstinvolves assessing the total dietary intake of the micr<strong>on</strong>utrient of interest, <strong>with</strong>and <strong>with</strong>out c<strong>on</strong>sidering the c<strong>on</strong>sumpti<strong>on</strong> of the fortified <strong>food</strong>. This allows thepercentage of the populati<strong>on</strong>, analysing each of the target groups independently(e.g. preschool-aged children, adolescents, women), that moves from havingintakes that are below the relevant EAR to having intakes that are above the EARto be estimated. The proporti<strong>on</strong> of the populati<strong>on</strong> that moves from below toabove the EAR provides a measure of the success of the programme. The sec<strong>on</strong>dapproach is to estimate the additi<strong>on</strong>al intake that would be supplied throughc<strong>on</strong>sumpti<strong>on</strong> of the fortified <strong>food</strong>. In this case, the measure of the programme’ssuccess is given by the proporti<strong>on</strong> of the populati<strong>on</strong> fulfilling that additi<strong>on</strong>alintake. Success criteria will inevitably vary according to the specific objectivesof the programme and should be set accordingly. However, it can be helpful toset stricter criteria for measuring coverage of targeted fortificati<strong>on</strong> programmes,in terms of the proporti<strong>on</strong> of the populati<strong>on</strong> that will benefit, to ensure that thosemost in need of fortified <strong>food</strong>s actually receive them.195
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS8.4 Impact evaluati<strong>on</strong>The main purpose of evaluating any interventi<strong>on</strong> is to determine whether or notit is reaching its overall goals. In the case of <strong>food</strong> fortificati<strong>on</strong> programmes, theprimary objective is to improve the nutriti<strong>on</strong>al status of the target populati<strong>on</strong>.Impact evaluati<strong>on</strong>s of most health and nutriti<strong>on</strong> programmes, including <strong>food</strong>fortificati<strong>on</strong> interventi<strong>on</strong>s, are however rarely performed, in part because theyare perceived as being complex, costly and sometimes threatening. The resultsof impact evaluati<strong>on</strong>s are nevertheless important decisi<strong>on</strong>-making tools, providinganswers to important questi<strong>on</strong>s such as:• Has the intake of a specific fortified <strong>food</strong> increased to expected levelsfollowing a <strong>food</strong> fortificati<strong>on</strong> programme?• Has the intake of specific nutrients of interest increased to expected levelsfollowing a <strong>food</strong> fortificati<strong>on</strong> programme?• Has the nutriti<strong>on</strong>al status of specific groups (for selected nutrients) improved,as a result of the fortificati<strong>on</strong> programme?• Has the fortificati<strong>on</strong> programme reduced the prevalence of specific micr<strong>on</strong>utrientdeficiencies?• Has the fortificati<strong>on</strong> programme reduced the prevalence of poor functi<strong>on</strong>aloutcomes, such as growth faltering, morbidity from infectious diseases, childmortality, and poor cognitive and motor development?• Has the fortificati<strong>on</strong> programme been more effective in improving status ofcertain micr<strong>on</strong>utrients and/or am<strong>on</strong>g certain age/physiological groups thanothers?The following subsecti<strong>on</strong>s review a range of methodologies that can be used for<strong>food</strong> fortificati<strong>on</strong> programme evaluati<strong>on</strong>, highlighting in each case the purposesand settings for which they are most appropriate. Although not all fortificati<strong>on</strong>programme evaluati<strong>on</strong>s will necessarily require the more sophisticated andtherefore the more costly methodologies, impartiality is always vital. In order toensure that impact evaluati<strong>on</strong>s are impartial, it is recommended that they arecarried out under the auspices of independent research groups or internati<strong>on</strong>alagencies. Ideally, funds for m<strong>on</strong>itoring and evaluati<strong>on</strong>, should be allocated at thetime of programme design and budget allocati<strong>on</strong>, but this is not to say that fundscannot be complemented by d<strong>on</strong>or agencies at a later date.8.4.1 Impact evaluati<strong>on</strong> designThere are a number of different ways in which the evaluati<strong>on</strong> of the impactof a <strong>food</strong> fortificati<strong>on</strong> programme can be tackled. However, the choice of196
8. MONITORING AND EVALUATIONmethodology should be dictated by the specific purpose of the evaluati<strong>on</strong>, andby the availability of resources.The level of precisi<strong>on</strong> required to satisfy the needsof decisi<strong>on</strong>-makers regarding the effectiveness of their programme is anotherimportant factor to bear in mind when selecting an evaluati<strong>on</strong> design.Habicht et al. (344) have devised a useful way of classifying the variousapproaches to evaluating public health interventi<strong>on</strong>s. The classificati<strong>on</strong> is based<strong>on</strong> the premise that the choice of the evaluati<strong>on</strong> method depends <strong>on</strong> the precisi<strong>on</strong>of data required by decisi<strong>on</strong>-makers to be able to say that the programmebeing evaluated has been effective. Three levels of inference are identified:adequacy, plausibility and probability. Applicati<strong>on</strong> of this classificati<strong>on</strong> to fortificati<strong>on</strong>programme evaluati<strong>on</strong> is presented in Table 8.5.8.4.1.1 Adequacy evaluati<strong>on</strong>An adequacy evaluati<strong>on</strong> is the appropriate choice if the objective is to assesswhether the prevalence of a particular micr<strong>on</strong>utrient deficiency is at or below apre-determined level. For example, the goal of a fortificati<strong>on</strong> programme maybe to reduce the prevalence of ir<strong>on</strong> deficiency am<strong>on</strong>g children to 10% or less(or any other cut-off point used to define a public health problem). In this case,adequacy would be achieved if the evaluati<strong>on</strong> showed that the prevalence of ir<strong>on</strong>deficiency at the time of the evaluati<strong>on</strong> was lower than the 10% pre-establishedcut-off point. Similarly, if a programme sought to raise the level of intake of fortifiedwheat flour by a target populati<strong>on</strong> group to a certain pre-determined level,an adequacy evaluati<strong>on</strong> would simply have to dem<strong>on</strong>strate that this level (or ahigher level) of intake has been reached by the targeted populati<strong>on</strong>.Adequacy evaluati<strong>on</strong>s are the simplest (and least costly) type of evaluati<strong>on</strong> tocarry out, primarily because they do not require randomizati<strong>on</strong> or the use of ac<strong>on</strong>trol group (Table 8.5). Nevertheless, adequacy evaluati<strong>on</strong>s demand the samelevel of scientific rigour as any other type of evaluati<strong>on</strong>. Appropriate studydesigns for this type of evaluati<strong>on</strong> include <strong>on</strong>e-time cross-secti<strong>on</strong>al surveys thatfocus <strong>on</strong> the outcome of interest.8.4.1.2 Plausibility evaluati<strong>on</strong>A plausibility evaluati<strong>on</strong> seeks to dem<strong>on</strong>strate, <strong>with</strong> a given level of certainty, thatthe reducti<strong>on</strong> in say, the prevalence of ir<strong>on</strong> deficiency, is related to the fortificati<strong>on</strong>programme being evaluated. Many factors unrelated to <strong>food</strong> fortificati<strong>on</strong>can reduce the prevalence of ir<strong>on</strong> deficiency, and thus the reducti<strong>on</strong> can bewr<strong>on</strong>gly attributed to the fortificati<strong>on</strong> programme unless the evaluati<strong>on</strong> takesthese factors into c<strong>on</strong>siderati<strong>on</strong>. For example, if public health measures toc<strong>on</strong>trol parasites and infecti<strong>on</strong>s have been implemented, or if development programmesto raise incomes have resulted in an increased intake of animal productsin the targeted populati<strong>on</strong>, a failure to c<strong>on</strong>trol for these external effects could197
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 8.5Evaluating the impact of fortificati<strong>on</strong> programmes <strong>on</strong> nutriti<strong>on</strong>al status: a range of approachesEvaluati<strong>on</strong> Aim of the evaluati<strong>on</strong> Evaluati<strong>on</strong> design requirementstypeAdequacy To assess whether the prevalence of Adequacy evaluati<strong>on</strong>s require a cross-secti<strong>on</strong>al survey of nutrient intakes, or of clinical,specific micr<strong>on</strong>utrient deficiencies functi<strong>on</strong>al or biochemical indicators of deficiency, at a certain point in time.(or the intake of specific Prevalence data must be evaluated against established criteria of adequacy, or of a publicmicr<strong>on</strong>utrients) is acceptable or health problem.such that there is a public health Assessment should focus <strong>on</strong> deficiencies in those micr<strong>on</strong>utrients that are of primary interest,problem. and which can be supplied in fortified <strong>food</strong>s.Plausibility To be able to state that it is plausible Plausibility evaluati<strong>on</strong>s require a quasi-experimental design such as:that <strong>food</strong> fortificati<strong>on</strong> was the — a cross-secti<strong>on</strong>al study which compares households (or individuals) that c<strong>on</strong>sumedcause of changes in nutriti<strong>on</strong>al fortified <strong>food</strong>s <strong>with</strong> a comparable group that did not;status. — a l<strong>on</strong>gitudinal study in which measures are recorded in the same individuals before andafter a period of fortificati<strong>on</strong>;— a l<strong>on</strong>gitudinal study in which measures are recorded before and after a period offortificati<strong>on</strong> in a group that received fortified <strong>food</strong>s, and also in a c<strong>on</strong>trol group that didnot; this allows changes due to other factors (e.g. <strong>food</strong> prices, nati<strong>on</strong>al ec<strong>on</strong>omy) to beaccounted for;— a case–c<strong>on</strong>trol study which compares cases who c<strong>on</strong>sumed fortified <strong>food</strong>s <strong>with</strong> c<strong>on</strong>trolswho did not but who are similar in many relevant characteristics, such as socioec<strong>on</strong>omicstatus, place of residence (i.e. geographic locati<strong>on</strong>, urban vs. rural, householdcompositi<strong>on</strong>), gender, age (i.e. matched c<strong>on</strong>trols).Probability To determine, <strong>with</strong> a level of Probability evaluati<strong>on</strong>s require a double-blind, randomized, experimental design that comparesprobability that was established resp<strong>on</strong>ses to fortified <strong>food</strong>s <strong>with</strong> n<strong>on</strong>-fortified <strong>food</strong>s. This requires:before the evaluati<strong>on</strong>, that — randomizati<strong>on</strong> of participants in the “fortified” and “n<strong>on</strong>-fortified” groups;observed changes in nutriti<strong>on</strong>al — before-and-after measurements in the same subjects;status are due to fortificati<strong>on</strong>. — that neither the participants nor the evaluators know which treatments are beingc<strong>on</strong>sumed by whom, during the interventi<strong>on</strong> or during the data analysis (i.e. adouble-blind study).198
8. MONITORING AND EVALUATIONwr<strong>on</strong>gly attribute the reducti<strong>on</strong> in ir<strong>on</strong> deficiency to <strong>food</strong> fortificati<strong>on</strong>. It is thereforeimportant for plausibility evaluati<strong>on</strong>s to c<strong>on</strong>trol for these potential c<strong>on</strong>foundingfactors and biases through the careful selecti<strong>on</strong> of an appropriate studydesign and through the use of multivariate data analysis techniques. Plausibilityevaluati<strong>on</strong>s use quasi-experimental or case–c<strong>on</strong>trol designs (Table 8.5): they requireeither the comparis<strong>on</strong> between an interventi<strong>on</strong> and a c<strong>on</strong>trol group (whodid not receive the interventi<strong>on</strong>), or before-and-after informati<strong>on</strong> <strong>on</strong> a groupwho received the interventi<strong>on</strong> (a pre–post design), or both (i.e. before-and-afterinformati<strong>on</strong> <strong>on</strong> both an interventi<strong>on</strong> and a c<strong>on</strong>trol group).8.4.1.3 Probability evaluati<strong>on</strong>Probability evaluati<strong>on</strong>s provide the highest level of c<strong>on</strong>fidence that the <strong>food</strong> fortificati<strong>on</strong>programme is resp<strong>on</strong>sible for the observed reducti<strong>on</strong> in the prevalenceof deficiency. Only probability methods can establish causality; these necessitatethe use of randomized, c<strong>on</strong>trolled experiments, carried out in a double-blindmanner whenever possible (Table 8.5). The probability evaluati<strong>on</strong> is based <strong>on</strong>the premise that there is <strong>on</strong>ly a small known probability (usually P < 0.05, i.e.a less than 5% chance) that the observed differences in ir<strong>on</strong> deficiency (forexample) between the group that was randomly assigned to receive fortified<strong>food</strong>s and the n<strong>on</strong>-fortified <strong>food</strong> c<strong>on</strong>trol group are due to chance.Probability evaluati<strong>on</strong>s are complex and expensive to perform because theyneed a randomized sample and a c<strong>on</strong>trol group. They may not be feasible inusual field c<strong>on</strong>diti<strong>on</strong>s, either for practical reas<strong>on</strong>s or for ethical reas<strong>on</strong>s. Forexample, if the fortified product is different in appearance and/or taste, it willbe impossible to carry out the interventi<strong>on</strong> in a double-blind manner. Similarly,it may not be practical to randomize the populati<strong>on</strong> into a <strong>food</strong>-fortified and ac<strong>on</strong>trol group. Moreover, using a c<strong>on</strong>trol group often raises ethical c<strong>on</strong>cerns.For these reas<strong>on</strong>s, probability methods are more comm<strong>on</strong>ly used for small, pilotefficacy trials (i.e. interventi<strong>on</strong>s carried out under c<strong>on</strong>trolled c<strong>on</strong>diti<strong>on</strong>s to determineefficacy), than for effectiveness trials (large-scale interventi<strong>on</strong>s carried outunder real-life field c<strong>on</strong>diti<strong>on</strong>s, and facing usual implementati<strong>on</strong> c<strong>on</strong>straints).Probability evaluati<strong>on</strong>s are the reference standard of efficacy research.Note that the questi<strong>on</strong>s listed above (page 14) assume either a plausibility ora probability evaluati<strong>on</strong> design, rather than an adequacy design. This is becausethe formulati<strong>on</strong> of these questi<strong>on</strong>s implies a change or an improvement that isattributable to the fortificati<strong>on</strong> programme. Adequacy evaluati<strong>on</strong> designs canaddress similar questi<strong>on</strong>s, but they would have to be phrased <strong>with</strong> reference topre-established criteria of adequacy, rather than <strong>with</strong> respect to a change attributableto the programme. For example, the first questi<strong>on</strong>:Has the intake of a specific fortified <strong>food</strong> increased to expected levels followinga <strong>food</strong> fortificati<strong>on</strong> programme?199
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSwould become:Is the intake of a specific fortified <strong>food</strong> at the expected level (say, is 90% ofthe populati<strong>on</strong> c<strong>on</strong>suming salt fortified at the mimimum household level)?Adequacy criteria could also be expressed in terms of biochemical indicators;for instance:Is the prevalence of vitamin A deficiency am<strong>on</strong>g preschool-aged childrenlower than say 20% (or any other pre-established criteria) following the <strong>food</strong>fortificati<strong>on</strong> programme?8.4.2 Methodological c<strong>on</strong>siderati<strong>on</strong>s8.4.2.1 Selecti<strong>on</strong> of outcome indicatorsOutcome indicators that can be used to assess the impact of fortificati<strong>on</strong> programmesinclude measures of intake (which can also be used as indicators ofutilizati<strong>on</strong> – see secti<strong>on</strong> 8.3; Table 8.4); clinical and biochemical indicators ofnutriti<strong>on</strong>al status (see Tables 3.1, 3.4, 3.6, 4.1, 4.3–4.5, 4.7, 4.8, 4.10, 4.11,4.13–4.16); and functi<strong>on</strong>al indicators such as growth, morbidity, mortality ordevelopment. Examples of each type of outcome indicator are given in Table8.6, al<strong>on</strong>g <strong>with</strong> suitable methods for their measurement.Given that the goal of <strong>food</strong> fortificati<strong>on</strong> is to improve the nutriti<strong>on</strong>al status ofa populati<strong>on</strong>, biochemical markers would normally be the indicators of choicefor evaluating the impact of fortificati<strong>on</strong> programmes. However, the measurementof biochemical status indicators requires c<strong>on</strong>siderable resources and technicalexpertise, for example, for collecting blood samples in the field and forc<strong>on</strong>ducting high-quality laboratory analyses, which means that this is not alwaysa practical, or indeed a feasible, opti<strong>on</strong>. Fortunately, there are cheaper and lesscomplex alternatives to measuring biochemical status indicators for assessingthe impact of a programme, such as measuring the c<strong>on</strong>sumpti<strong>on</strong> of a fortifiedproduct or the intake of a particular micr<strong>on</strong>utrient of interest. These measuresare suitable alternatives to biochemical indicators in cases where str<strong>on</strong>g evidenceof their validity has been obtained from either rigorous efficacy trials 1 or fromeffectiveness trials c<strong>on</strong>ducted in similar c<strong>on</strong>diti<strong>on</strong>s as the programme being evaluated.For example, if it has been established in efficacy trials that c<strong>on</strong>sumpti<strong>on</strong>of a certain minimum amount of a given fortified product results in a desirablechange in <strong>on</strong>e or more biochemical indicators (and prevents micr<strong>on</strong>utrient deficiency),other fortificati<strong>on</strong> programmes using the same <strong>food</strong> vehicle can rely <strong>on</strong>1An efficacy trial is <strong>on</strong>e that applies an interventi<strong>on</strong> under c<strong>on</strong>trolled c<strong>on</strong>diti<strong>on</strong>s to determine themagnitude of effect that can be achieved under the best possible circumstances (344). Effectivenesstrials, <strong>on</strong> the other hand, test the impact of an interventi<strong>on</strong> under real life c<strong>on</strong>diti<strong>on</strong>s, andgiven usual operati<strong>on</strong>al inefficiencies that occur under normal field c<strong>on</strong>diti<strong>on</strong>s.200
8. MONITORING AND EVALUATIONTABLE 8.6Impact evaluati<strong>on</strong> of a <strong>food</strong> fortificati<strong>on</strong> programme: suggested outcomemeasuresOutcome measureMethodology and resp<strong>on</strong>sible entityIntake indicatorsMethod: Any of the following dietary assessmentmethods:Adequate or increased intake — weighed intake;of fortified <strong>food</strong>(s) a— 24-hour recall;— <strong>food</strong> frequency questi<strong>on</strong>naire;Adequate or increased intake — assessment of usual intake.of specific micr<strong>on</strong>utrient(s) Resp<strong>on</strong>sible: Independent researchers.of interestNutriti<strong>on</strong>al status indicators Method: Recommended biochemical and clinicalAdequate or improvedindicators for selected micr<strong>on</strong>utrients are listed inbiochemical and clinical Tables 3.1, 3.4, 3.6, 4.1, 4.3–4.5, 4.7, 4.8, 4.10, 4.11,status indicators for 4.3–4.16.micr<strong>on</strong>utrient(s) of interest Resp<strong>on</strong>sible: Independent researchers.Functi<strong>on</strong>al outcomesMethod: Standard approaches to the measurement ofAdequate outcomes orthese functi<strong>on</strong>al outcomes should be used, for example:improvements in functi<strong>on</strong>al — for growth, anthropometry;outcomes such as growth, — for morbidity, 2-week recall or surveillance data;morbidity, mortality and — for mortality, recall data;motor and cognitive— for child cognitive and motor development, thedevelopmentappropriate battery of tests and scales.Resp<strong>on</strong>sible: Independent researchers.abM<strong>on</strong>itoring of the intake of fortified products can also be d<strong>on</strong>e as part of household m<strong>on</strong>itoring(see Table 8.4).An impact evaluati<strong>on</strong> should <strong>on</strong>ly be performed <strong>on</strong>ce programme m<strong>on</strong>itoring has indicatedthat programme is operating in a satisfactory manner and therefore, is in theory capable ofachieving its nutriti<strong>on</strong>al goals. Full impact evaluati<strong>on</strong> need <strong>on</strong>ly be d<strong>on</strong>e <strong>on</strong>ce, as l<strong>on</strong>g asregular m<strong>on</strong>itoring ensures appropriate fortificati<strong>on</strong> levels at all stages (i.e. in factories, retailstores and households), adequate utilizati<strong>on</strong> of the product and adequate coverage of thetargeted populati<strong>on</strong>.c<strong>on</strong>sumpti<strong>on</strong> data to measure their impact. This technique is comm<strong>on</strong>ly employedin evaluati<strong>on</strong>s of salt iodizati<strong>on</strong> and immunizati<strong>on</strong> programmes. In thecase of the former, coverage informati<strong>on</strong> is used to measure success, an approachthat is valid because there is str<strong>on</strong>g evidence that iodized salt, c<strong>on</strong>sumed regularlyand in sufficient amounts, is effective in preventing iodine deficiency. Theselecti<strong>on</strong> of outcome indicators is discussed further in secti<strong>on</strong> 8.5 in the c<strong>on</strong>textof the minimum requirements for m<strong>on</strong>itoring and evaluati<strong>on</strong> systems for fortificati<strong>on</strong>interventi<strong>on</strong>s.8.4.2.2 Data requirementsIn order to be able to c<strong>on</strong>duct an impact evaluati<strong>on</strong> using any of the indicatorsand methodologies listed in Table 8.6, it is necessary to first calculate the201
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSnumber of subjects that will need to be surveyed (i.e. the sample size) so as toensure a result of adequate precisi<strong>on</strong> and sensitivity (i.e. be able to detect differencesof a particular size when they exist). Ideally, a random procedure shouldbe used to select subjects for study.Specific data needs for each category of impact evaluati<strong>on</strong> are as follows:• Adequacy evaluati<strong>on</strong>s require data <strong>on</strong> the chosen outcomes, and also aminimum amount of informati<strong>on</strong> about the study subjects (such as age, sexand physiological status) to facilitate interpretati<strong>on</strong> of the results.• Plausibility evaluati<strong>on</strong>s demand more detailed informati<strong>on</strong> about the studysubjects in order to account for c<strong>on</strong>founding factors. However, the moreinformati<strong>on</strong> that is collected <strong>on</strong> possibly c<strong>on</strong>founding or other explanatoryfactors, the more rigorous the evaluati<strong>on</strong> design will need to be if it is todem<strong>on</strong>strate that the outcome achieved is related to the interventi<strong>on</strong>. It istherefore prudent to collect informati<strong>on</strong> <strong>on</strong> factors unrelated to the fortificati<strong>on</strong>programme, but which may have c<strong>on</strong>tributed to the changes observed inthe outcome of interest. Data from other programmes implemented in thearea, <strong>on</strong> say community improvements, and <strong>on</strong> household and individualsociodemographic characteristics can all help strengthen the analysis andinterpret the findings. This type of informati<strong>on</strong> can also be used to understandpathways and mechanisms, and to help interpret lack of impact.• For probability evaluati<strong>on</strong>s, if a double-blind experimental study design isused, c<strong>on</strong>trol for c<strong>on</strong>founding influences is not required. However, it is alwaysuseful to have informati<strong>on</strong> <strong>on</strong> intermediary outcomes to help describe mechanismsand dose–resp<strong>on</strong>se relati<strong>on</strong>ships, and to identify subgroups of thepopulati<strong>on</strong> that may have benefited more (or less) than others from theinterventi<strong>on</strong>.8.4.2.3 Timing of an impact evaluati<strong>on</strong>As noted at the start of this chapter, an evaluati<strong>on</strong> of the impact of a fortificati<strong>on</strong>programme should not be undertaken until a certain level of operati<strong>on</strong>alperformance has been achieved. Say, for example, commercial m<strong>on</strong>itoring establishesthat levels of a micr<strong>on</strong>utrient in a product available from retail stores are<strong>on</strong>ly 20% of what they should be, c<strong>on</strong>ducting an impact evaluati<strong>on</strong> of such apoorly functi<strong>on</strong>ing programme would <strong>on</strong>ly be a waste of time, effort and m<strong>on</strong>ey.It is therefore important that fortificati<strong>on</strong> programmes establish a priori theminimum criteria for the quality of service delivery that it must achieve beforeany efforts to evaluate its impact are undertaken.The timing of programme evaluati<strong>on</strong> is will also depend <strong>on</strong> how quickly animpact <strong>on</strong> the biochemical indicators of interest can be expected. In other words,how so<strong>on</strong> after a programme has been implemented and has been found to be202
8. MONITORING AND EVALUATIONoperating satisfactorily should an impact evaluati<strong>on</strong> be undertaken? Both thetype of interventi<strong>on</strong> (fortificati<strong>on</strong> versus supplementati<strong>on</strong>, for example) and thenutrient(s) of interest are key factors to c<strong>on</strong>sider. In relati<strong>on</strong> to the former,the amount of nutrient(s) delivered daily in fortified <strong>food</strong>s is usually much lessthan that which can be administered in a supplement; moreover, the fortified<strong>food</strong>s may not be c<strong>on</strong>sumed every day, or in the expected amounts. The combinedeffect of these factors is that it will take l<strong>on</strong>ger for the biological impactof a micr<strong>on</strong>utrient fortificati<strong>on</strong> programme to become detectable than it will fora supplementati<strong>on</strong> programme, probably by as much as several m<strong>on</strong>ths (especiallyin the case of effectiveness trials). For instance, it takes about 6–9 m<strong>on</strong>thsbefore the effect of ir<strong>on</strong> fortificati<strong>on</strong> <strong>on</strong> ir<strong>on</strong> status is seen.The rate of change in nutriti<strong>on</strong>al status indicators varies substantially by nutrient,and also according to the sensitivity of the indicator. It takes about 1–2 yearsfrom the start of a salt iodizati<strong>on</strong> programme to see a significant reducti<strong>on</strong> ingoitre. Some individuals may take even l<strong>on</strong>ger than this to recover, especially ifthey are also ir<strong>on</strong> deficient (86). On the other hand, urinary iodine is a fairlyresp<strong>on</strong>sive indicator of iodine intake, and should increase significantly <strong>with</strong>in afew weeks of the commencement of an increased iodine c<strong>on</strong>sumpti<strong>on</strong>. On thewhole, changes in biochemical indicators of vitamin status tend to be more rapidthan those in indicators of mineral status. For instance, populati<strong>on</strong> serum folateand plasma homocysteine c<strong>on</strong>centrati<strong>on</strong>s resp<strong>on</strong>d <strong>with</strong>in 6 m<strong>on</strong>ths of the introducti<strong>on</strong>into the diet of flour fortified <strong>with</strong> folic acid (49,52). Similarly, c<strong>on</strong>sumpti<strong>on</strong>of sugar fortified <strong>with</strong> vitamin A produces measurable impacts after<strong>on</strong>ly 6 m<strong>on</strong>ths (46).8.4.2.4 Counfounding factorsFinally, when planning an impact evaluati<strong>on</strong> it is important to recognize that anumber of factors can affect the ability of individuals to resp<strong>on</strong>d to fortificati<strong>on</strong>.Particularly significant in this regard is the prevalence of parasitic infestati<strong>on</strong>sand infecti<strong>on</strong>s in a populati<strong>on</strong>. Some parasites cause large, c<strong>on</strong>tinuing micr<strong>on</strong>utrientlosses; hookworm, for example, causes intestinal loss of blood and thereforeincreased losses of ir<strong>on</strong>, vitamin A, vitamin B 12 and several other nutrients.Parasite c<strong>on</strong>trol programmes are obviously an effective strategy in these situati<strong>on</strong>sand should be instigated in c<strong>on</strong>juncti<strong>on</strong> <strong>with</strong> <strong>food</strong> fortificati<strong>on</strong>.The presence of parasites and infecti<strong>on</strong>s can also affect the sensitivity of indicatorsof nutriti<strong>on</strong>al status, which can make the impact of a fortificati<strong>on</strong> programmemore difficult to detect. For example, haemoglobin and serum ferritinare resp<strong>on</strong>sive to changes in ir<strong>on</strong> status but are also affected by inflammati<strong>on</strong>and infectious disorders. If these c<strong>on</strong>diti<strong>on</strong>s are widespread, ir<strong>on</strong> status can<strong>on</strong>ly really be assessed using a combinati<strong>on</strong> of indicators, that is serumferritin in combinati<strong>on</strong> <strong>with</strong> serum transferrin receptors or erythrocyte zinc203
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSprotoporphyrine, and an indicator of inflammati<strong>on</strong>, such as C-reative protein(75) (see also Table 3.1). This approach has been adopted <strong>with</strong> good effect inboth Viet Nam (28) and in Morocco (44) to dem<strong>on</strong>strate the efficacy of ir<strong>on</strong>fortificati<strong>on</strong> of fish sauce and salt, respectively. The presence of malaria presentsparticular challenges: malaria not <strong>on</strong>ly leads to a substantial reducti<strong>on</strong> inhaemoglobin c<strong>on</strong>centrati<strong>on</strong>s, but also affects many other nutriti<strong>on</strong>al status indicators,including serum ferritin, serum transferrin and transferrin receptors,plasma retinol and erythrocyte riboflavin (152). Simultaneous assessment ofmalaria parasites (by blood smears) or more accurately, of malaria antigens usingtest strips (152), and of indicators of inflammati<strong>on</strong> (such as alpha-1 glycoprotein,and C-reactive protein), will assist in the detecti<strong>on</strong> of individuals whose testresults may be affected by malaria.8.5 What is the minimum every fortificati<strong>on</strong> programme shouldhave in terms of a m<strong>on</strong>itoring and evaluati<strong>on</strong> system?This chapter has highlighted the importance for <strong>food</strong> fortificati<strong>on</strong> programmesof having a well-planned m<strong>on</strong>itoring and evaluati<strong>on</strong> system. These systemsshould be designed in such a way that the informati<strong>on</strong> provided by m<strong>on</strong>itoringand evaluati<strong>on</strong> is used effectively for decisi<strong>on</strong>-making and for overall programmemanagement. In order for this to happen, resp<strong>on</strong>sibilities for data collecti<strong>on</strong> atthe different levels must be clearly established and the system must include feedbackloops, which allow the informati<strong>on</strong> to flow (in a timely manner) to the entitiesresp<strong>on</strong>sible for taking acti<strong>on</strong> at the different levels.Regulatory m<strong>on</strong>itoring is an essential part of any m<strong>on</strong>itoring and evaluati<strong>on</strong>system and should always be implemented, at least to some degree. Informati<strong>on</strong>from internal, external and commercial m<strong>on</strong>itoring activities should be sharedregularly <strong>with</strong> all sectors engaged in the <strong>food</strong> fortificati<strong>on</strong> programme. Feedbackactivities should include the sharing of informati<strong>on</strong> about successes and anyfollow up <strong>on</strong> corrective measures required when problems were detected.Of equal importance is household m<strong>on</strong>itoring. Its value lies in its ability toprovide a general appraisal of the impact of the programme, and in the absenceof an effective system of nutriti<strong>on</strong>al surveillance, it also provides informati<strong>on</strong>about the importance of <strong>food</strong> fortificati<strong>on</strong> in the diet of target populati<strong>on</strong>s. Theannual cost of household m<strong>on</strong>itoring has been estimated at less than US$10 000 per fortified <strong>food</strong> (O. Dary, pers<strong>on</strong>al communicati<strong>on</strong>, 2004). Despiteits relatively low cost, household m<strong>on</strong>itoring is often neglected in manyprogrammes. In many settings, household m<strong>on</strong>itoring is dependent <strong>on</strong>external d<strong>on</strong>ors for financial support, a factor which limits its permanence andsustainability.The chapter has also stressed the urgent need to measure the impact of <strong>food</strong>fortificati<strong>on</strong> programmes, again to support decisi<strong>on</strong>-making, and, in particular,204
8. MONITORING AND EVALUATIONto assist programme planners and policy-makers in making decisi<strong>on</strong>s about programmec<strong>on</strong>tinuati<strong>on</strong>, modificati<strong>on</strong>, expansi<strong>on</strong> or terminati<strong>on</strong>. Different typesof impact evaluati<strong>on</strong>s can be employed; these vary in their level of sophisticati<strong>on</strong>and in the intensity of resources required. Decisi<strong>on</strong>s about which specifictype of evaluati<strong>on</strong> and which outcome indicators to use should be driven primarilyby programme objectives and the level of precisi<strong>on</strong> required to be ableto attribute impact to the programme itself (i.e. this will determine whether anadequacy or a more complex plausibility design is needed, for example).The choice of outcome indicator(s) for impact evaluati<strong>on</strong>s is a pivotal <strong>on</strong>e.Questi<strong>on</strong>s that can help guide the selecti<strong>on</strong> of an appropriate of outcomeindicator are:• Can intake measures be used instead of more invasive (and often more costly)biochemical indicators?• How often do impact evaluati<strong>on</strong>s have to be carried out?The answer to these questi<strong>on</strong>s largely depends <strong>on</strong> the availability and strengthof evidence from efficacy trials and previous effectiveness evaluati<strong>on</strong>s of comparableprogrammes c<strong>on</strong>ducted in similar envir<strong>on</strong>ments and populati<strong>on</strong> groups.The results of <strong>on</strong>ly <strong>on</strong>e or just a few efficacy trials are usually sufficient to provethat a fortified <strong>food</strong> can change the nutriti<strong>on</strong>al status (and its associated biologicalindicators) in a human populati<strong>on</strong>, in which case it might not be necessaryto repeat such experiments in each community (see also secti<strong>on</strong> 8.4.2.1).It thus follows that the first step in planning an impact evaluati<strong>on</strong> is usually todetermine whether or not there is str<strong>on</strong>g evidence from existing efficacy trialsthat the planned interventi<strong>on</strong> causes a given impact when c<strong>on</strong>ducted underc<strong>on</strong>trolled c<strong>on</strong>diti<strong>on</strong>s.If str<strong>on</strong>g evidence from efficacy trials can be established, effectiveness trialscan then be implemented to test whether the same impact can also be achievedwhen the interventi<strong>on</strong> is delivered under normal field c<strong>on</strong>diti<strong>on</strong>s and programmec<strong>on</strong>straints. In the case of fortificati<strong>on</strong> programmes, if other effectiveness trialsindicate that an impact can be obtained over a given period of time <strong>with</strong> anintake of a specific amount of micr<strong>on</strong>utrients through the c<strong>on</strong>sumpti<strong>on</strong> of fortifiedproducts, there is no need to invest in the more resource intensive andcomplex dem<strong>on</strong>strati<strong>on</strong>s of impact <strong>on</strong> biochemical indicators. It may be sufficientto ensure that the targeted populati<strong>on</strong> c<strong>on</strong>sumes the fortified <strong>food</strong> ofexpected quality in sufficient amounts and <strong>with</strong> adequate frequency. However,before c<strong>on</strong>clusi<strong>on</strong>s obtained from <strong>on</strong>e community can be extrapolated toanother, it is important to assure that the c<strong>on</strong>diti<strong>on</strong>s are similar. It may be necessaryto c<strong>on</strong>duct efficacy trials to corroborate findings <strong>on</strong>ce every 5–10 years,especially if envir<strong>on</strong>mental, dietary and health c<strong>on</strong>diti<strong>on</strong>s of the targeted populati<strong>on</strong>change rapidly. This objective might be combined <strong>with</strong> the functi<strong>on</strong> of205
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSgeneral nutriti<strong>on</strong> surveys to m<strong>on</strong>itor the evoluti<strong>on</strong> of the nutriti<strong>on</strong>al status of thepopulati<strong>on</strong>.Obviously, in the absence of str<strong>on</strong>g evidence from efficacy trials, there are noshort-cuts and a detailed impact evaluati<strong>on</strong> (efficacy trial or probability evaluati<strong>on</strong>),involving appropriate biochemical indicators, will need to be carried out.The comments made previously about the timing of evaluati<strong>on</strong>s (secti<strong>on</strong>8.4.2.3) and the need to c<strong>on</strong>sider potential c<strong>on</strong>founding factors (secti<strong>on</strong> 8.4.2.4)become especially pertinent in these circumstances.Summary■ A well-designed, well-managed m<strong>on</strong>itoring and evaluati<strong>on</strong> system is essential forensuring the success and sustainability of any <strong>food</strong> fortificati<strong>on</strong> programme. As integralcomp<strong>on</strong>ents of the programme, m<strong>on</strong>itoring and evaluati<strong>on</strong>s activities should beformulated and budgeted for during the very early planning stages.■ Some degree of regulatory m<strong>on</strong>itoring is critical. Of the three main categories ofregulatory m<strong>on</strong>itoring – internal m<strong>on</strong>itoring (c<strong>on</strong>ducted at factories and packers),external m<strong>on</strong>itoring (c<strong>on</strong>ducted at factories and packers) and commercial m<strong>on</strong>itoring(c<strong>on</strong>ducted at retail stores) – internal m<strong>on</strong>itoring is a must. In settings whereeffective enforcement mechanisms exist, it is usually sufficient to c<strong>on</strong>firm compliance<strong>with</strong> regulati<strong>on</strong>s in samples taken from retail stores (commercial m<strong>on</strong>itoring).Elsewhere it is prudent to c<strong>on</strong>duct external m<strong>on</strong>itoring at both the factory level andat retail stores.■ Impact evaluati<strong>on</strong>s should <strong>on</strong>ly be carried <strong>on</strong>ce it has been established, throughregulatory and household m<strong>on</strong>itoring, that the programme has achieved a predeterminedlevel of operati<strong>on</strong>al efficiency.■ Although rigorous impact evaluati<strong>on</strong>s of <strong>food</strong> fortificati<strong>on</strong> programmes are urgentlyneeded, not all programmes will require the most costly and sophisticated designs.Judicious choices will have to be made in selecting the most appropriate evaluati<strong>on</strong>for each particular situati<strong>on</strong>.206
CHAPTER 9Estimating the cost-effectiveness andcost–benefit of fortificati<strong>on</strong>Not<strong>with</strong>standing the limitati<strong>on</strong>s menti<strong>on</strong>ed in secti<strong>on</strong> 1.4, <strong>food</strong> fortificati<strong>on</strong> mayoften be the least expensive way of achieving a particular nutriti<strong>on</strong>al goal, suchas a specified reducti<strong>on</strong> in the prevalence of anaemia, iodine deficiency or subclinicalvitamin A deficiency. Put another way, fortificati<strong>on</strong> is frequently morecost-effective than other public health interventi<strong>on</strong>s that have the potential toachieve the same health or nutriti<strong>on</strong>al outcome, such as supplementati<strong>on</strong>.Indeed, several studies have dem<strong>on</strong>strated that fortificati<strong>on</strong> is not <strong>on</strong>ly costeffective(i.e. is a cheaper way to increase micr<strong>on</strong>utrient intake compared <strong>with</strong>other interventi<strong>on</strong>s that have the same aim), but also has a high cost–benefit ratio(i.e. is a good investment).In this chapter the c<strong>on</strong>cepts of cost-effectiveness and cost–benefit are formallydefined. Techniques for estimating the cost-effectiveness of an interventi<strong>on</strong>and for performing a cost–benefit analysis are also outlined, and illustratedin the latter half of the chapter by a series of example calculati<strong>on</strong>s for a hypotheticallow-income country. The methods employed can be readily modifiedand applied to other countries. Although both cost-effectiveness and cost–benefit analyses are widely used as decisi<strong>on</strong>-making tools by policy-makersworking in the public health arena, their applicati<strong>on</strong> to <strong>food</strong> fortificati<strong>on</strong> is a relativelynew development. To date, <strong>on</strong>ly interventi<strong>on</strong>s involving ir<strong>on</strong>, iodine andvitamin A have been evaluated in these terms, and c<strong>on</strong>sequently form the focusof the material presented here.9.1 Basic c<strong>on</strong>cepts and definiti<strong>on</strong>s9.1.1 Cost-effectivenessCost-effectiveness is defined as the cost of achieving a specified outcome. In thecase of <strong>food</strong> fortificati<strong>on</strong>, examples of the desired outcome might include: averting<strong>on</strong>e case of subclinical vitamin A deficiency, averting <strong>on</strong>e case of anaemia,or averting <strong>on</strong>e case of goitre or of iodine deficiency.Two outcome measures that are frequently employed in cost-effectivenessassessments of health interventi<strong>on</strong>s are the “cost per death averted” and the “costper disability-adjusted life-year saved” (or cost per DALY saved). The former,the cost per death averted, has been successfully used to assess the cost-207
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSeffectiveness of various fortificati<strong>on</strong> and supplementati<strong>on</strong> interventi<strong>on</strong>s, but inthis c<strong>on</strong>text its applicati<strong>on</strong> requires making various critical assumpti<strong>on</strong>s (seesecti<strong>on</strong> 9.2.1). For example, costs per death averted have been estimated forvitamin A supplementati<strong>on</strong> for children and for ir<strong>on</strong> supplementati<strong>on</strong> for pregnantwomen (groups that are particularly susceptible to deficiencies and thereforefrequently targeted in interventi<strong>on</strong> programmes). However, it is a less usefulcalculati<strong>on</strong> in the case of iodine fortificati<strong>on</strong>, principally because mortalityoutcomes are relatively rare, the main benefit being increased productivity (seesecti<strong>on</strong> 9.3.2).The advantage of the other widely used effectiveness measure, the cost perDALY saved, lies in the fact that it combines mortality and morbidity outcomesinto a single indicator (354,355).This measure has been employed to good effectto assess the effectiveness of various health interventi<strong>on</strong>s, including fortificati<strong>on</strong>and supplementati<strong>on</strong>, as part of WHO’s CHOICE project (see Box 9.1).However, relative to the alternative measure, the cost per death averted, its calculati<strong>on</strong>is more demanding in terms of data requirements and the assumpti<strong>on</strong>sthat must be made (see secti<strong>on</strong> 9.2.1)Cost-effectiveness analysis is a particularly useful exercise for comparing differentinterventi<strong>on</strong>s that share the same outcome, for example, for comparingsupplementati<strong>on</strong> <strong>with</strong> vitamin A <strong>with</strong> fortificati<strong>on</strong> <strong>with</strong> vitamin A, or for comparingvitamin A supplementati<strong>on</strong> <strong>with</strong> immunizati<strong>on</strong>. In both cases the sharedoutcome is the number of deaths averted.The two pieces of informati<strong>on</strong> requiredfor the calculati<strong>on</strong> of the cost-effectiveness of an interventi<strong>on</strong> are: the unit costof the interventi<strong>on</strong> (i.e. the cost per pers<strong>on</strong> assisted per year), and some measureof the effect of the interventi<strong>on</strong> (i.e. the proporti<strong>on</strong> of the target populati<strong>on</strong> thatachieves some specified outcome). The cost estimates, being less resource intensive,tend to be easier to obtain than the estimates of the effect, which require(at a minimum) a baseline and a follow-up assessment, and (ideally) a c<strong>on</strong>trolgroup.BOX 9.1Choosing interventi<strong>on</strong>s that are cost-effective:WHO’s CHOICE Pro j e c tCHOICE stands for “CHOosing Interventi<strong>on</strong>s that are Cost-Effective”, and is atool developed by WHO to help decisi<strong>on</strong>-makers select those interventi<strong>on</strong>s andprogrammes that provide the maximize benefits for the available resources. Bygeneralizing the cost-effectiveness analysis, the applicati<strong>on</strong> of the CHOICEmodel indicates which interventi<strong>on</strong>s provide the best value for m<strong>on</strong>ey.Applicati<strong>on</strong> of the CHOICE model to data from WHO’s Africa D regi<strong>on</strong>(mainly West Africa) has dem<strong>on</strong>strated that micr<strong>on</strong>utrient interventi<strong>on</strong>s are208
9. ESTIMATING THE COST-EFFECTIVENESS AND COST–BENEFIT OF FORTIFICATIONpotentially highly cost-effective 1 . In Figure 9.1 the average cost per DALY savedby hypothetical programmes for zinc supplementati<strong>on</strong> in the under-fives(coverage, 80% of the target populati<strong>on</strong>), ir<strong>on</strong> supplementati<strong>on</strong> in pregnantwomen (coverage, 50% of pregnant women), vitamin A/zinc fortificati<strong>on</strong>(coverage, 80% of the general populati<strong>on</strong>), and ir<strong>on</strong> fortificati<strong>on</strong> (coverage,80% of the general populati<strong>on</strong>) are compared. Both the fortificati<strong>on</strong> programmesachieve relatively low costs per DALY saved. The same ir<strong>on</strong> and vitamin A/zincfortificati<strong>on</strong> programmes are compared in Figure 9.2, but this time <strong>with</strong> thefollowing interventi<strong>on</strong>s: oral rehydrati<strong>on</strong> (coverage, 80% of the target populati<strong>on</strong>),case management of pneum<strong>on</strong>ia (coverage, 80% of the target populati<strong>on</strong>),and disinfecti<strong>on</strong> of water supply at point of use combined <strong>with</strong> wateruse educati<strong>on</strong> (coverage, 100% target populati<strong>on</strong>). Whereas all of theseprogrammes were found to be highly cost-effective, the fortificati<strong>on</strong> programmeswere particularly so.FIGURE 9.1Cost-effectiveness of micr<strong>on</strong>utrient supplementati<strong>on</strong> and fortificati<strong>on</strong>100$/DALY saved806040200Zincsupplementati<strong>on</strong>Ir<strong>on</strong>supplementati<strong>on</strong>Vitamin A/Zinc fortificati<strong>on</strong>Ir<strong>on</strong>fortificati<strong>on</strong>Interventi<strong>on</strong>FIGURE 9.2Cost-effectiveness of selected interventi<strong>on</strong>s affecting children250$/DALY saved200150100500Ir<strong>on</strong>fortificati<strong>on</strong>Vitamin A/Zinc fortificati<strong>on</strong>Oralrehydrati<strong>on</strong>Pneum<strong>on</strong>iamanagementDisinfecti<strong>on</strong> ofwater supplyInterventi<strong>on</strong>1Further informati<strong>on</strong> about the CHOICE project, including a descripti<strong>on</strong> of the methodologyemployed, can be found <strong>on</strong> the WHO web site at: http://www.who.int/choice/en/.209
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS9.1.2 Cost–benefit analysisCost-effectiveness analyses are valuable tools for comparing interventi<strong>on</strong>s thatshare the same outcome; if however, the objective is to compare interventi<strong>on</strong>s<strong>with</strong> different outcomes, or to compare interventi<strong>on</strong>s whose potential benefitsor outcomes extend bey<strong>on</strong>d health, then a cost–benefit analysis is needed. In itssimplest form, a cost–benefit analysis compares the m<strong>on</strong>etary cost of an interventi<strong>on</strong><strong>with</strong> the m<strong>on</strong>etary value of the outcome (i.e. the benefit). The outcomesor benefits may be increased productivity (e.g. ir<strong>on</strong> fortificati<strong>on</strong> makes adultsless anaemic and hence more productive) or possibly lower health care systemcosts (e.g. mothers who are less anaemic will incur fewer complicati<strong>on</strong>s duringchildbirth). Since cost–benefit analyses can be used to compare the relativemerits of health interventi<strong>on</strong>s <strong>with</strong> other kinds of government spending, they areespecially helpful for advocating for increased resources for nutriti<strong>on</strong> and health.A cost–benefit ratio calculati<strong>on</strong> requires much the same unit cost and effectdata as a cost-effectiveness analysis. Again, the cost data are typically easier andcheaper to obtain than the effect data. In additi<strong>on</strong>, the benefit or rather theoutcome of a health interventi<strong>on</strong> (e.g. a reducti<strong>on</strong> in prevalence of goitre or achange in the mean urinary iodine excreti<strong>on</strong> of a populati<strong>on</strong>) has to be expressedin financial terms, that is to say, assigned a m<strong>on</strong>etary value. Most cost–benefitstudies do not do this directly, but rely <strong>on</strong> the findings of other studies that havelinked the proximate health outcome to a financial benefit. For example,cost–benefit analyses involving iodine interventi<strong>on</strong>s, which are seeking to estimatethe financial gain of eliminating <strong>on</strong>e case of goitre (as an intermediateoutcome), turn to studies that have estimated the costs associated <strong>with</strong> the lossof productivity per child born to a mother <strong>with</strong> goitre. The worked example presentedin secti<strong>on</strong> 9.3.2 adopts this approach.9.2 Informati<strong>on</strong> needs9.2.1 Estimating unit costsUnit cost calculati<strong>on</strong>s (i.e. the calculati<strong>on</strong> of the cost of the interventi<strong>on</strong> perpers<strong>on</strong> per year) need to take into account not just the recurrent costs of supplyingfortificants or supplements, but also a number of other associated costs.For fortificati<strong>on</strong>, these typically include:—the initial investment in the technology required for adding the fortificantto the <strong>food</strong> vehicle (which will vary depending <strong>on</strong> the number of processingfacilities and the existing level of technology, the micr<strong>on</strong>utrient andthe nature of the packaging, storage and/or handling of the final productthat is required);— the cost of “social marketing” to attain public acceptance of (or preferencefor) the fortified <strong>food</strong>;210
9. ESTIMATING THE COST-EFFECTIVENESS AND COST–BENEFIT OF FORTIFICATION— the cost of quality c<strong>on</strong>trol and quality assurance by producers and of governmentm<strong>on</strong>itoring and evaluati<strong>on</strong> activities.In the case of supplementati<strong>on</strong>, the additi<strong>on</strong>al costs may include the time andlogistics costs of the distributi<strong>on</strong> of the supplement (which are not alwaysreported), and again, the costs associated <strong>with</strong> m<strong>on</strong>itoring and evaluati<strong>on</strong>.Typical costs incurred by a wheat flour fortificati<strong>on</strong> programme (<strong>with</strong> ir<strong>on</strong> andzinc) are set out in Table 9.1; these include the initial investment costs (amortizedover expected lifetime of the equipment), recurrent costs, and the cost ofm<strong>on</strong>itoring and evaluati<strong>on</strong>.The estimated unit costs of various past supplementati<strong>on</strong> and fortificati<strong>on</strong>programmes, compiled by Levin et al. (357), are listed in Table 9.2. Accordingto these data, unit costs for supplementati<strong>on</strong> are c<strong>on</strong>sistently higher than thosefor fortificati<strong>on</strong>. Supplementati<strong>on</strong> costs are 10–30 times higher than fortificati<strong>on</strong>costs in the case of iodine, 3–30 times higher for ir<strong>on</strong>, and 1.5–3 timeshigher for vitamin A. The cost differential is largely dependent <strong>on</strong> what proporti<strong>on</strong>the target populati<strong>on</strong> is of the whole populati<strong>on</strong>; fortificati<strong>on</strong> becomesincreasingly cost-effective the higher the proporti<strong>on</strong> of the populati<strong>on</strong> in needof the interventi<strong>on</strong>.Although now rather out of date, the unit cost data reported by Levin et al.(357) do provide some useful insight into the relative cost-effectiveness of supplementati<strong>on</strong>and fortificati<strong>on</strong> as strategies for correcting micr<strong>on</strong>utrient deficiencies.For instance, in the case of vitamin A, if supplementati<strong>on</strong> costs 2–2.5times as much as fortificati<strong>on</strong> per pers<strong>on</strong>, supplementati<strong>on</strong> is potentially themore attractive opti<strong>on</strong> when the target group comprises less than 40–50% of thepopulati<strong>on</strong> (e.g. children aged less than 2 years). However, for ir<strong>on</strong> the situati<strong>on</strong>is reversed: per pers<strong>on</strong> ir<strong>on</strong> supplementati<strong>on</strong> is at least 10 times more costlythan fortificati<strong>on</strong> but the prevalence of anaemia is well over 10% in most developingcountry populati<strong>on</strong>s. In this case then, mass fortificati<strong>on</strong> would most likelybe the more cost-effective strategy. It should be stressed that these c<strong>on</strong>clusi<strong>on</strong>sare based <strong>on</strong> average data and cannot be applied to all settings; the relative costeffectivenessof supplementati<strong>on</strong> and fortificati<strong>on</strong> will vary markedly acrosscountries according to both the unit cost of the interventi<strong>on</strong> and the fracti<strong>on</strong> ofpopulati<strong>on</strong> targeted.Another factor to c<strong>on</strong>sider in the supplementati<strong>on</strong> versus fortificati<strong>on</strong> debateis the effectiveness of the interventi<strong>on</strong> itself; this can be highly variable. In thecase of vitamin A deficiencies, both supplementati<strong>on</strong> and fortificati<strong>on</strong> have beenshown to be effective in impact evaluati<strong>on</strong>s (33,46). In areas of endemic iodinedeficiency, salt iodizati<strong>on</strong> programmes have also been shown to be highly effective(25,359). However, the evidence for the effectiveness of ir<strong>on</strong> interventi<strong>on</strong>sis less clear cut (see secti<strong>on</strong> 1.3.1.1). Recently completed studies fromChina and Viet Nam, involving soy and fish sauces, respectively, suggest that211
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE 9.1Hypothetical annual costs of wheat flour fortificati<strong>on</strong> <strong>with</strong> ir<strong>on</strong> and zinc(assumes an annual flour producti<strong>on</strong> of 100 000 t<strong>on</strong>nes at 1 mill using ac<strong>on</strong>tinuous fortificati<strong>on</strong> system)Cost of ir<strong>on</strong> Additi<strong>on</strong>al cost Total costsfortificati<strong>on</strong> of including zinc (US$)(US$)(US$)Industry costsCapital investment 820 0 820Recurrent costsEquipment (maintenance, depreciati<strong>on</strong>) 600 0 600Ferrous sulfate fortificant a 57 090 NA 57 090Zinc sulfate fortificant b NA 102 600 102 600Quality c<strong>on</strong>trol 7 920 2 880 c 10 800Total industry costs 66 430 105 480 171 910Industry costs per t<strong>on</strong>ne fortified wheat flour 0.66 1.05 1.72State costsCapital investment and maintenance 2 625 0 2 625Mill inspecti<strong>on</strong> and m<strong>on</strong>itoringSalaries and transportati<strong>on</strong> 3 500 0 3 500Laboratory analysis and reports (including 1 500 96 d 1 596technician salaries)Quality assurance and m<strong>on</strong>itoring training 1 000 500 e 1 500Programme m<strong>on</strong>itoring (i.e. dietary intake,travel, per diems, analysis, reports) 1 400 0 1 400Evaluati<strong>on</strong>Travel, per diems, collecti<strong>on</strong> of biological 3 000 0 3 000samplesLaboratory analysis and reports (including 5 000 3 600 f 8 600technician salaries)Total state costs 18 025 4 196 22 221Total programme costs 84 455 109 676 194 131Total cost per t<strong>on</strong>ne fortified wheat flour 0.84 1.10 1.94Total cost per capita (assuming an intake of 0.05 0.06 0.11150 g per pers<strong>on</strong> per day)aCost of the ferrous sulfate (US$ 8.65/kg (pure ir<strong>on</strong>)), plus an additi<strong>on</strong>al 33% to allow for shippingcosts, added to 100 000 t<strong>on</strong>nes wheat flour at 66 ppm.bCost of the zinc sulfate (US$ 34.20/kg (pure zinc)), plus an additi<strong>on</strong>al 33% to allow for shippingcosts, added to 100 000 t<strong>on</strong>nes wheat flour at 30 ppm.cAssuming 2 samples are analysed per day, for 360 days per year at a cost of US$ 4 persample.dAssuming 1 sample per m<strong>on</strong>th is collected from the marketplace and analysed in duplicate,for 12 m<strong>on</strong>ths of the year at a cost of US$ 4 per sample.eAn additi<strong>on</strong>al 50% of the cost of quality assurance and m<strong>on</strong>itoring training was included tocover the zinc assessment.fProgramme evaluati<strong>on</strong> based <strong>on</strong> serum zinc analysis in a sample of 1 500 preschool-agedchildren: assuming a cost of US$ 4 per sample, and a total of three assessments c<strong>on</strong>ductedin a 5 year period (i.e. baseline, after 12–15 m<strong>on</strong>ths, and 5 years post-programme initiati<strong>on</strong>),the cost is US$ 18 000 over the 5-year period or US$ 3 600 per annum.Source: adapted from reference (356).212
9. ESTIMATING THE COST-EFFECTIVENESS AND COST–BENEFIT OF FORTIFICATIONTABLE 9.2Estimated unit costs of selected micr<strong>on</strong>utrient interventi<strong>on</strong>sInterventi<strong>on</strong> Country, year of Cost per Cost per Cost per pers<strong>on</strong>programme pers<strong>on</strong> pers<strong>on</strong> per year of(US$) (1987 US$) protecti<strong>on</strong>(1987 US$) aIodineOil injecti<strong>on</strong> Zaire, 1977 0.35 0.67 0.14Oil injecti<strong>on</strong> Peru, 1978 1.30 2.30 0.46Oil injecti<strong>on</strong> Bangladesh, 1983 0.70 0.76 0.25Oil injecti<strong>on</strong> Ind<strong>on</strong>esia, 1986 1.00 1.05 0.21Salt fortificati<strong>on</strong> India, 1987 0.02–0.04 0.02–0.04 0.02–0.04Water fortificati<strong>on</strong> Italy, 1986 0.04 0.04 0.04Vitamin ASugar fortificati<strong>on</strong> Guatemala, 1976 0.07 0.14 0.14Capsule Ind<strong>on</strong>esia/ 0.10 0.21 0.42Philippines, 1975Capsule Haiti, 1978 0.13–0.19 0.23–0.34 0.46–0.68Capsule Bangladesh, 1983 0.05 0.05 0.10Ir<strong>on</strong>Salt fortificati<strong>on</strong> India, 1980 0.07 0.10 0.10Sugar fortificati<strong>on</strong> Guatemala, 1980 0.07 0.10 0.10Sugar fortificati<strong>on</strong> Ind<strong>on</strong>esia, 1980 0.60 0.84 0.84Tablets Kenya/Mexico, 1980 1.89–3.17 2.65–4.44 2.65–4.44aDifferent interventi<strong>on</strong>s supply vitamin and mineral requirements for different lengths of time.The cost per year has therefore been adjusted to take account of these differences in thedurati<strong>on</strong> of protecti<strong>on</strong> provided by the interventi<strong>on</strong>.Sources: references (357,358).fortificati<strong>on</strong> <strong>with</strong> NaFeEDTA has been instrumental in reducing ir<strong>on</strong> deficiencyanaemia am<strong>on</strong>g women (28). On the other hand, despite the fact that ir<strong>on</strong> supplementati<strong>on</strong>has proved to be efficacious in c<strong>on</strong>trolled trials (360), many ir<strong>on</strong>supplementati<strong>on</strong> programmes have been relatively ineffective in improvinganaemia status, even in targeted subgroups. One possible explanati<strong>on</strong> for this isapparent discrepancy is that in many cases ir<strong>on</strong> deficiency is not the main causeof the observed anaemia, but rather it is some other factor.9.2.2 Cost-effectiveness analysesMost cost-effectiveness analyses rely <strong>on</strong> a single indicator or outcome measureto reflect the change brought about by the interventi<strong>on</strong>, usually a measure ofnutriti<strong>on</strong>al status. However, in terms of the magnitude of the calculated costeffectiveness,different outcome measures do not always yield the same result.Possible outcome indicators for ir<strong>on</strong>, for example, include the change in meanhaemoglobin level, the change in mean haemoglobin level of the initially213
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSdeficient populati<strong>on</strong>, and the proporti<strong>on</strong> of the populati<strong>on</strong> removed fromanaemia. The first measure gives equal weight to improvements in haemoglobinstatus irrespective of the initial level of deficiency, the sec<strong>on</strong>d gives equal weightto all those initially deficient (again, irrespective whether the deficiency wassevere or mild), and the third will give a higher weight to improvements in themildly deficient, but will ignore improvements that d<strong>on</strong>’t “bump” people overthe threshold, even if their haemoglobin status improves. (As explained inChapter 3, anaemia is an imperfect indicator of ir<strong>on</strong> status due to the fact thatin many populati<strong>on</strong>s anaemia has multiple causes).The most useful outcome or effect measures for cost-effectiveness analysestend to be those which also provide informati<strong>on</strong> <strong>on</strong> the causes of the change innutriti<strong>on</strong>al status. This is particularly helpful when making comparis<strong>on</strong>s <strong>with</strong>other studies, which may have employed a different outcome measure. Ifrestricted to using <strong>on</strong>ly a single outcome measure, then it is desirable to selectthe <strong>on</strong>e that can be linked to other outcomes of interest. In the ir<strong>on</strong> exampleabove, the proporti<strong>on</strong> of the populati<strong>on</strong> removed from anaemia is the mostuseful effect indicator, because it is possible to link anaemia status (i.e.anaemic/not anaemic) to productivity outcomes or to pregnancy complicati<strong>on</strong>outcomes.The cost-effectiveness of fortificati<strong>on</strong> interventi<strong>on</strong>s is likely to vary c<strong>on</strong>siderablyaccording to the prevailing c<strong>on</strong>diti<strong>on</strong>s, since it is heavily dependent <strong>on</strong> thefollowing factors:• the <strong>food</strong> vehicle used, the storage c<strong>on</strong>diti<strong>on</strong>s and the stability of the fortificantduring storage;• the initial level of deficiency in the populati<strong>on</strong> (e.g. improvements in ir<strong>on</strong>status may be easier to obtain in initially more deficient populati<strong>on</strong>s, becausetheir ir<strong>on</strong> absorpti<strong>on</strong> is more efficient and because the cost per case ofanaemia averted is lower if more of the populati<strong>on</strong> is anaemic);• dietary patterns, especially <strong>with</strong> respect to the c<strong>on</strong>sumpti<strong>on</strong> of <strong>food</strong>s whichinhibit or enhance absorpti<strong>on</strong> of the micr<strong>on</strong>utrient of interest in the samemeal;• marketing and processing patterns, and whether the chosen vehicle is c<strong>on</strong>sumedby all households in the groups likely to be deficient, including thepoor and those living in remote areas.Despite the inherent variability in the cost-effectiveness of <strong>food</strong> fortificati<strong>on</strong>interventi<strong>on</strong>s, it is not necessary to perform analyses for all programmes and forall c<strong>on</strong>diti<strong>on</strong>s. Nevertheless, informati<strong>on</strong> should be obtained for a selecti<strong>on</strong> ofprogrammes operating under a range of c<strong>on</strong>diti<strong>on</strong>s.214
9. ESTIMATING THE COST-EFFECTIVENESS AND COST–BENEFIT OF FORTIFICATION9.2.3 Cost–benefit analysisUndertaking a cost–benefit analysis of a fortificati<strong>on</strong> programme is generallymore involved and certainly more demanding of data (and assumpti<strong>on</strong>s) thanis a cost-effectiveness analysis. However, <strong>on</strong>ly cost–benefit analyses permit comparis<strong>on</strong>sacross a broad range of benefits, including n<strong>on</strong>-health outcomes. Issuesto bear in mind when undertaking cost–benefit analysis include the following:• What benefits should be included? Some benefits (e.g. lower health care costsbecause of improved ir<strong>on</strong> status, and thus reduced numbers of maternaldeaths) may be important, but hard to calculate in the developing countryc<strong>on</strong>text. Omitting important benefits will make the results more c<strong>on</strong>servative.• Should n<strong>on</strong>-market benefits be taken into account? The effects of <strong>food</strong> fortificati<strong>on</strong>,for example, improved productivity in women, will <strong>on</strong>ly partiallyshow up as market benefits. Fortificati<strong>on</strong> may well result in important n<strong>on</strong>marketbenefits, such as better child-care, which will affect the market productivityof the next generati<strong>on</strong>. Ideally then, n<strong>on</strong>-market benefits should bevalued, by using shadow prices or c<strong>on</strong>tingent valuati<strong>on</strong> methods.• How can future benefits be incorporated? Ideally, the present value of thefuture benefits stream should be included, appropriately discounted, say by3% (the social rate of discount typically employed in cost–benefit-type analyses).Nevertheless, even this low rate of discount still favours interventi<strong>on</strong>s<strong>with</strong> immediate benefits (e.g. those targeted at adults) relative to those <strong>with</strong>future benefits (e.g. those targeted at children).• Cost–benefit analysis (unless equity weights are used) tends to favour interventi<strong>on</strong>sthat benefit the rich more than the poor (the rich have higher wages,and c<strong>on</strong>sequently higher productivity losses when they die or fall ill), and similarlythose benefiting men rather than women (as men are the more ec<strong>on</strong>omicallyproductive, at least in terms of market benefits).• Because of the assumpti<strong>on</strong>s required, it is sometimes desirable to present theresults of a cost–benefit analysis in natural units (e.g. in terms of productivity(for ir<strong>on</strong>-deficiency anaemia) or morbidity rates (for vitamin A deficiency)as well as in m<strong>on</strong>etary values.It is possible to undertake cost–benefit analyses prospectively (i.e. incidencestudies), but this necessitates making assumpti<strong>on</strong>s about how a new fortificati<strong>on</strong>programme will affect the future time path of outcomes, discounting all costsand benefits to the present (361). The alternative is a prevalence study, in whichcosts of fortificati<strong>on</strong> are compared <strong>with</strong> the existing costs attributable to deficiency.The latter requires fewer assumpti<strong>on</strong>s, is simpler to undertake, and maybe quite useful for advocacy purposes (see Chapter 10). In the series of worked215
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSexamples presented in these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>, a prevalence method has been used toestimate the cost–benefit ratio of interventi<strong>on</strong>s to correct deficiencies of iodineand ir<strong>on</strong> (see secti<strong>on</strong>s 9.3.2 and 9.3.3).9.3 Estimating the cost-effectiveness and cost–benefit ofvitamin A, iodine and ir<strong>on</strong> interventi<strong>on</strong>s: worked examplesFor the purposes of illustrating of applicati<strong>on</strong> of cost-effectiveness andcost–benefit analysis methodologies to <strong>food</strong> fortificati<strong>on</strong>, example calculati<strong>on</strong>sare set out below for three micr<strong>on</strong>utrients, namely, vitamin A, iodine and ir<strong>on</strong>.Country-specific data required to perform these calculati<strong>on</strong>s is given in Table9.3 for a hypothetical large, low-income developing country P. These data wouldbe needed to replicate the cost–benefit and cost-effectiveness calculati<strong>on</strong>s foranother country. Use of generally accepted fortificati<strong>on</strong> costs (i.e. those set outin Table 9.3 and which are derived from historical programme data) is recommended,unless country-specific data are available.The sample calculati<strong>on</strong>s require several key assumpti<strong>on</strong>s to be made c<strong>on</strong>cerningthe ec<strong>on</strong>omic c<strong>on</strong>sequences of deficiency (Table 9.4). Assumpti<strong>on</strong>smust also be made about the effectiveness of a given fortificati<strong>on</strong> programme.Although it is clear that effectiveness of fortificati<strong>on</strong> depends <strong>on</strong> the chosen <strong>food</strong>TABLE 9.3Country-specific data required for cost-effectiveness and cost–benefitcalculati<strong>on</strong>s, country PAnnual per capita GDP US$ 430Child death rate 117.4 per 1 000Proporti<strong>on</strong> of children ≤5 years in populati<strong>on</strong> 25.6%Share of labour force in agriculture 25%Prevalence of subclinical vitamin A deficiency, children ≤5 years 30%Cost per pers<strong>on</strong> per year of vitamin A fortificati<strong>on</strong> US$ 0.10Prevalence of goitre, women of childbearing age 15%Cost per pers<strong>on</strong> per year of iodine fortificati<strong>on</strong> US$ 0.10Prevalence of anaemia (populati<strong>on</strong> average) 37.25%Cost per pers<strong>on</strong> per year of ir<strong>on</strong> fortificati<strong>on</strong> US$ 0.12Infant mortality rate 80 per 1 000Maternal mortality rate 200 per 100 000Cost per pregnancy of ir<strong>on</strong> supplementati<strong>on</strong> US$ 1.70For the purposes of illustrating of applicati<strong>on</strong> of cost-effectiveness and cost–benefit analysismethodologies to <strong>food</strong> fortificati<strong>on</strong>, example calculati<strong>on</strong>s are set out below for three micr<strong>on</strong>utrients,namely, vitamin A, iodine and ir<strong>on</strong>. Country-specific data required to perform these calculati<strong>on</strong>sis given in Table 9.3 for a hypothetical large, low-income developing country P. Thesedata would be needed to replicate the cost–benefit and cost-effectiveness calculati<strong>on</strong>s foranother country. Use of generally accepted fortificati<strong>on</strong> costs (i.e. those set out in Table 9.3 andwhich are derived from historical programme data) is recommended, unless country-specificdata are available.216
9. ESTIMATING THE COST-EFFECTIVENESS AND COST–BENEFIT OF FORTIFICATIONTABLE 9.4Key assumpti<strong>on</strong>s in estimating cost-effectiveness and cost–benefit ofselected micr<strong>on</strong>utrient fortificati<strong>on</strong>Mic<strong>on</strong>utrient Assumpti<strong>on</strong>s Reference(s)Vitamin A The relative risk of mortality for children <strong>with</strong> subclinical (362)vitamin A deficiency (compared <strong>with</strong> those that aren<strong>on</strong>-deficient) is <strong>on</strong> average 1.75 : 1.Iodine Of all births to women <strong>with</strong> goitre, 3.4% are cretins (103,104,355,363)(productivity loss 100%), 10.2% are severelymentally impaired (productivity loss 25%), andthe rest suffer minor IQ loss (productivity loss 5%).Ir<strong>on</strong> Productivity loss associated <strong>with</strong> anaemia is 5% (361)(light manual work), 17% (heavy manual work)and 4% in all other kinds of work.The odds ratio associated <strong>with</strong> 10 g/l increase in (364)haemoglobin is 0.80 for maternal mortality, and0.72 for perinatal mortality inAfrica (0.84 in other regi<strong>on</strong>s); prenatalsupplementati<strong>on</strong> <strong>with</strong> ir<strong>on</strong> is associated <strong>with</strong>11.7 g/l improvement in haemoglobin.vehicle, the compositi<strong>on</strong> of the usual diet, and the pre-existing level of deficiencyin the populati<strong>on</strong>, it is rarely possible to accurately account for such variati<strong>on</strong>s,due to a lack of field data. Under such circumstances, it is instructive to c<strong>on</strong>ducta sensitivity analysis, according to the key assumpti<strong>on</strong>s made. This involvesrepeating the calculati<strong>on</strong>s several times, varying each of the key parametersin turn. If the cost-effectiveness ratio does not change dramatically, or thecost–benefit ratio remains robust (i.e. benefits remain large relative to costs), asthe parameters are changed, then greater c<strong>on</strong>fidence can be placed in thec<strong>on</strong>clusi<strong>on</strong>s.9.3.1 Vitamin A supplementati<strong>on</strong>: a cost-effectiveness calculati<strong>on</strong>Cost–benefit calculati<strong>on</strong>s cannot readily be undertaken for interventi<strong>on</strong>s involvingvitamin A. Although there are subsequent productivity effects, the moreimmediate benefit of vitamin A supplementati<strong>on</strong> in children is a reducti<strong>on</strong>in child morbidity and mortality. For this reas<strong>on</strong>, it is rather more helpful toestimate the cost-effectiveness of vitamin A fortificati<strong>on</strong> or supplementati<strong>on</strong>(expressed as the cost per death averted or the cost per DALY saved), whichcan then be compared <strong>with</strong> other public health interventi<strong>on</strong>s that have the potentialto achieve the same outcome.The calculati<strong>on</strong> of the cost-effectiveness of vitamin A fortificati<strong>on</strong>, using thecost per death averted as the outcome measure, hinges <strong>on</strong> the assumpti<strong>on</strong> that217
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSall child deaths due to vitamin A deficiency (VAD) can be averted by vitaminA fortificati<strong>on</strong>. If this assumpti<strong>on</strong> is made, the calculati<strong>on</strong> is simply a matter ofestimating the proporti<strong>on</strong> of all child deaths that are due to VAD, this beingequivalent to the number of deaths that can be averted by fortificati<strong>on</strong>.The populati<strong>on</strong> attributable risk due to vitamin A deficiency (PAR VAD ) 1 iscalculated from the prevalence of VAD in children and probability or risk ofdying from VAD, according to the following formula:where:PAR VAD = [Pre VAD × (RR VAD − 1)]/[1 + Pre VAD × (RR VAD − 1)]Pre VAD = the prevalence of vitamin A am<strong>on</strong>g childrenin the under-6 years age group; andRR VAD = the relative risk 2 of mortality for children <strong>with</strong> subclinical VAD.Then, based <strong>on</strong> the values given in Tables 9.3 and 9.4, in country P,PAR VAD = (0.3 × 0.75)/(1 + 0.3 × 0.75) = 0.183.In country P, the child death rate (i.e. in the under-fives) is 117.4 per 1000.Hence the number of child deaths per year that theoretically could be preventedby eliminating VAD in this populati<strong>on</strong> group is:0.183 × 117.4 = 21.48 per 1000.Suppose that the unit cost of vitamin A fortificati<strong>on</strong> per year is US$ 0.10. Thisrepresents the cost of providing 100% of the daily requirements of vitamin Afor the populati<strong>on</strong> in wheat flour, or 75% of the daily requirements of preschoolagedchildren via margarine (O. Dary, pers<strong>on</strong>al communicati<strong>on</strong>, 2004). If, incountry P, children under 5 years of age account for 25.6% of the populati<strong>on</strong>(Table 9.3), then the cost of fortificati<strong>on</strong> per child aged under 5 years is:0.10/0.256 = 0.39, or US$ 0.39 per year.The cost per death averted is therefore:12The populati<strong>on</strong> attributable risk (PAR) is defined as the proporti<strong>on</strong> of cases in the total populati<strong>on</strong>that are attributable to the risk factor.The relative risk (RR) is defined as the ratio of the probability of disease development am<strong>on</strong>gexposed individuals to the probability of disease development in n<strong>on</strong>-exposed individuals.218
9. ESTIMATING THE COST-EFFECTIVENESS AND COST–BENEFIT OF FORTIFICATION0.39/0.02148 = 18.16, or US$ 18.16 per year.This cost can then be compared <strong>with</strong> that of alternative interventi<strong>on</strong>s which savechildren’s lives, such as immunizati<strong>on</strong> and treatment of infectious disease. Thecosts per death averted for the latter are typically significantly higher, which suggeststhat vitamin A fortificati<strong>on</strong> would be a very cost-effective interventi<strong>on</strong> forreducing childhood mortality in country P.9.3.2 Iodine: a cost–benefit analysisIn the cost–benefit calculati<strong>on</strong> for iodine described here, goitre prevalence isused to indicate iodine deficiency and the main ec<strong>on</strong>omic c<strong>on</strong>sequence of iodinedeficiency is assumed to be productivity losses in those children born to mothers<strong>with</strong> goitre (see Table 9.4). Although in many respects urinary iodine excreti<strong>on</strong>is a better indicator of iodine deficiency (it tracks improvements in iodine intakemore rapidly (6), at present, such data are not widely available for many countries.Nor is the relati<strong>on</strong>ship between urinary iodine excreti<strong>on</strong> and birth outcomeswell documented, although it is anticipated that this will become clearerin the future.Based <strong>on</strong> the assumpti<strong>on</strong>s given in Table 9.4, the average percentage productivityloss per birth to a mother <strong>with</strong> goitre is:(100% × 0.034) + (25% × 0.102) + (5% × 0.864) = 10.27%.The per capita productivity loss in country P, where the prevalence of goitre inwomen is 15%, is given by the formula:Productivity loss per capita = Prevalence of goitre × Average productivityloss × Wage share in GDP × Per capita GDP.Note that instead of multiplying an average productivity loss by an averagewage expressed in units of currency, and applying a factor which equates tothe proporti<strong>on</strong> of the populati<strong>on</strong> that works in the market labour force, weuse here a simplificati<strong>on</strong>, as follows:We assume that the average wage in the populati<strong>on</strong> is given by:Average wage = (Per capita GDP × Wage share in GDP)/Employment proporti<strong>on</strong>,where the employment proporti<strong>on</strong> is the market labour force as a share ofthe total populati<strong>on</strong>.219
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSIf the wage share in GDP in country P is 40%, then applicati<strong>on</strong> of the aboveformula gives a per capita productivity loss of:0.15 × 0.1027 × 0.40 × 430 = 2.65, or US$ 2.65.If the unit cost of iodine fortificati<strong>on</strong> is US$ 0.10 per pers<strong>on</strong> per year (359),then the cost–benefit ratio of iodine fortificati<strong>on</strong> is 0.10 : 2.65 or 1 : 26.5. If thecosts of fortificati<strong>on</strong> are as low as US$ 0.01, as has been suggested by Dary(pers<strong>on</strong>al communicati<strong>on</strong>, 2004) for parts of central America, then thecost–benefit ratio will be even greater.This is a very favourable cost–benefit ratio.These calculati<strong>on</strong>s make the critical assumpti<strong>on</strong> that iodine fortificati<strong>on</strong> programmesare 100% effective, i.e. that they completely remove the possibility ofgoitre in the populati<strong>on</strong> in the l<strong>on</strong>g term.9.3.3 Ir<strong>on</strong> fortificati<strong>on</strong>: a cost–benefit analysisThe cost–benefit analysis for ir<strong>on</strong> outlined below uses the prevalence of anaemiaas a proxy indicator of ir<strong>on</strong> deficiency. However, it is generally accepted that<strong>on</strong>ly about half of the cases of anaemia are in fact ir<strong>on</strong>-deficiency anaemia; c<strong>on</strong>versely,there are a c<strong>on</strong>siderable number of ir<strong>on</strong> deficiency cases that are notassociated <strong>with</strong> anaemia (see secti<strong>on</strong> 3.1.1). Despite its being an imperfect indicatorof ir<strong>on</strong> deficiency, anaemia is nevertheless used in this analysis in theabsence of alternative inexpensive and easy-to-apply tests of ir<strong>on</strong> deficiency (seediscussi<strong>on</strong> in Ross & Hort<strong>on</strong> (365). The present cost–benefit calculati<strong>on</strong> furtherassumes that the main ec<strong>on</strong>omic effect of ir<strong>on</strong> deficiency is a loss of manualwork, i.e. productivity. Based <strong>on</strong> the assumpti<strong>on</strong>s given in Table 9.4, the productivityloss for a known prevalence of anaemia (Pre anaemia ) is given by theformula:Productivity loss associated <strong>with</strong> anaemia over all market work + Additi<strong>on</strong>alproductivity loss associated <strong>with</strong> anaemia in light manual labour + Furtheradditi<strong>on</strong>al productivity loss associated <strong>with</strong> anaemia in heavy manual labour,that is,4% × Wage share in GDP × Per capita GDP × Pre anaemia + 1% × Wage share inGDP × Per capita GDP × Pre anaemia × Light manual share + 12% × Wage sharein GDP × Per capita GDP × Pre anaemia × Heavy manual share.Although the prevalence of anaemia (Pre anemia ) is not necessarily c<strong>on</strong>gruent <strong>with</strong>presence of ir<strong>on</strong> deficiency, it is nevertheless an appropriate indicator to use heresince the estimates of productivity losses employed (see Table 9.4) are derived220
9. ESTIMATING THE COST-EFFECTIVENESS AND COST–BENEFIT OF FORTIFICATIONfrom studies involving ir<strong>on</strong> interventi<strong>on</strong>s in anaemic populati<strong>on</strong>s, not specificallyir<strong>on</strong>-deficient populati<strong>on</strong>s.According to statistics produced by the Internati<strong>on</strong>al Labour Organizati<strong>on</strong>(ILO), in low-income countries light manual labour represents about 70% of allmarket work, 60% in lower-middle income countries, and 50% in upper-middleincome countries (366). For the purposes of this calculati<strong>on</strong>, it can be assumedthat 57.5% of the work in agriculture is heavy manual labour (based <strong>on</strong> theassumpti<strong>on</strong> that half of work in agriculture and c<strong>on</strong>structi<strong>on</strong> is heavy manualwork, and that c<strong>on</strong>structi<strong>on</strong> represents 15% of work in agriculture (366).If in country P, the proporti<strong>on</strong> of employment in agriculture is 25%, the overallprevalence of anaemia in the populati<strong>on</strong> is 37.25%, and light manual labour represents60% of all market work (the country being in the lower-middle incomecategory), then the per capita productivity losses associated <strong>with</strong> ir<strong>on</strong> deficiencyare as follows:(4% × 0.4 × 430 × 0.3725) +(1% × 0.4 × 0.6 × 430 × 0.3725) +(12% × 0.4 × 0.144 × 430 × 0.3725)= 4.04 US$.For a unit fortificati<strong>on</strong> cost per pers<strong>on</strong> of US$ 0.12 (based <strong>on</strong> data fromVenezuela (39), this produces a cost–benefit ratio of 0.12 : 4.04. However, asmenti<strong>on</strong>ed above, ir<strong>on</strong> fortificati<strong>on</strong> cannot correct all anaemia (i.e. it is not 100%effective), and a further adjustment to account for this fact needs to be made.According to the Venezuelan study c<strong>on</strong>ducted by Layrisse et al. (39), ir<strong>on</strong> fortificati<strong>on</strong>led to a 9% reducti<strong>on</strong> in the prevalence of anaemia. However, the studywas limited to children aged 7, 11 and 15 years, and was based <strong>on</strong> a before-andaftercomparis<strong>on</strong>, rather than <strong>on</strong> an interventi<strong>on</strong>/c<strong>on</strong>trol design. Layrisse’s c<strong>on</strong>clusi<strong>on</strong>sare, however, supported by the results of a well-c<strong>on</strong>trolled study fromMorocco involving double-fortified salt (<strong>with</strong> ir<strong>on</strong> and iodine). In this case, fortificati<strong>on</strong>,albeit at a higher c<strong>on</strong>centrati<strong>on</strong>, achieved a 15% decline in the prevalenceof ir<strong>on</strong>-deficiency anaemia in children aged 6–14 years, for an estimatedannual cost of US$ 0.22 (44).If it is assumed that in country P the same absolute decrease in anaemia prevalencecan be obtained as was achieved in Venezuela (for the whole populati<strong>on</strong>,not <strong>on</strong>ly children), then the proporti<strong>on</strong>al reducti<strong>on</strong> in anaemia due to the fortificati<strong>on</strong>programme would be:0.09/0.3725, or 24%.So, if the ec<strong>on</strong>omic benefit of averting ir<strong>on</strong> deficiency in the populati<strong>on</strong> is US$4.04 per pers<strong>on</strong>, and the cost of fortificati<strong>on</strong> is US$ 0.12 per pers<strong>on</strong> and the221
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSeffectiveness is 24% (i.e. a fortificati<strong>on</strong> programme reduces the prevalence by24%), then the cost–benefit ratio becomes:0.12 : 4.04 × 0.24 or 1 : 8.This is a fairly high cost–benefit ratio, and suggests that ir<strong>on</strong> fortificati<strong>on</strong> wouldbe a prudent investment in country P. The cost–benefit ratio for ir<strong>on</strong> fortificati<strong>on</strong>is lower than that calculated for iodine (see previous secti<strong>on</strong>). However, ifbenefits are assessed in terms of reduced mortality (as opposed to productivitylosses), ir<strong>on</strong> fortificati<strong>on</strong> produces the better cost–benefit ratio. Additi<strong>on</strong>al benefitsfor both iodine and ir<strong>on</strong>, not taken into account here, include improvementsin cognitive development and in school performance in children.9.3.4 Ir<strong>on</strong> supplementati<strong>on</strong>: a cost-effectiveness calculati<strong>on</strong>Studies have established that ir<strong>on</strong> supplementati<strong>on</strong> during pregnancy is associated<strong>with</strong> a 11.7 g/l improvement in haemoglobin levels. In turn, a 10 g/limprovement in haemoglobin levels is associated <strong>with</strong> an odds ratio of 0.80 formaternal mortality rates (MMR), and an odds ratio of 0.84 for perinatal mortalityrates (taken as 40% of the infant mortality rate (IMR)). Based <strong>on</strong> thesedata (see Table 9.4), it can be assumed that ir<strong>on</strong> supplementati<strong>on</strong> in pregnancyproduces a reducti<strong>on</strong> in the MMR from 200/100 000 to 137/100 000 live births(or from 2 to 1.37 per 1000 live births), and a reducti<strong>on</strong> of the perinatal mortalityrate from 32 per 1000 to 23 per 1000 live births.Hence, for an investment of US$ 1700 per 1000 pregnancies, 9.63 deaths areaverted (9 perinatal deaths and 0.63 maternal mortalities). This equates to a costper death averted of US$ 176.5.While <strong>on</strong> the surface it appears that ir<strong>on</strong> supplementati<strong>on</strong> during pregnancyis a less cost-effective strategy than vitamin A fortificati<strong>on</strong> in children (the costper death averted is about 10 times higher – see secti<strong>on</strong> 9.3.1), it should beremembered that ir<strong>on</strong> supplementati<strong>on</strong> also has immediate productivity benefitsthat would not be c<strong>on</strong>ferred by vitamin A.222
9. ESTIMATING THE COST-EFFECTIVENESS AND COST–BENEFIT OF FORTIFICATIONSummaryThe cost-effectiveness of an interventi<strong>on</strong> is expressed in terms of the cost of achievinga specified outcome. Analyses of cost-effectiveness are particularly useful for comparingdifferent interventi<strong>on</strong>s that share the same outcome. In assessments of healthinterventi<strong>on</strong>s, the two most widely used effectiveness measures are “cost per deathaverted” and the “cost per disability adjusted life-year saved” (cost per DALY saved).Both measures can be applied to micr<strong>on</strong>utrient interventi<strong>on</strong>s. Although the lattermeasure combines mortality and morbidity outcomes into a single indicator, its calculati<strong>on</strong>is generally more demanding in terms of data needs and assumpti<strong>on</strong>s.A cost–benefit analysis compares the m<strong>on</strong>etary cost of an interventi<strong>on</strong> <strong>with</strong> the m<strong>on</strong>etaryvalue of a specified outcome (i.e. the benefit). Because cost–benefit analysesare able to compare interventi<strong>on</strong>s whose potential benefits or outcomes extend bey<strong>on</strong>dhealth, they can be used to evaluate the relative merits of health interventi<strong>on</strong>s andother kinds of government spending. Cost–benefit analyses are thus especially helpfulfor advocating for increased resources for nutriti<strong>on</strong> and health.Cost-effectiveness and cost-benefit analyses have shown that:■ Both iodine and ir<strong>on</strong> fortificati<strong>on</strong> have the potential to achieve high cost–benefitratios, given the prevailing levels of micr<strong>on</strong>utrient deficiency and the ec<strong>on</strong>omicsituati<strong>on</strong> of many low-income countries.■ Food fortificati<strong>on</strong> <strong>with</strong> vitamin A is highly cost-effective in reducing mortality in children,as is supplementati<strong>on</strong> <strong>with</strong> ir<strong>on</strong> in pregnant women.■ Fortificati<strong>on</strong> becomes increasingly cost-effective the higher the proporti<strong>on</strong> of thepopulati<strong>on</strong> in need of the interventi<strong>on</strong>.223
CHAPTER 10Communicati<strong>on</strong>, social marketing,& advocacy in support of <strong>food</strong>fortificati<strong>on</strong> programmesIn comm<strong>on</strong> <strong>with</strong> other health promoti<strong>on</strong> programmes, all <strong>food</strong> fortificati<strong>on</strong>programmes share two objectives:(i)to create an enabling envir<strong>on</strong>ment – in this case, <strong>on</strong>e that makes adequatelyfortified <strong>food</strong>s widely available and provides the means for individuals toacquire them;(ii) to help individuals adopt healthful behaviours – in this case, behaviours thatenhance the c<strong>on</strong>tributi<strong>on</strong> of fortified <strong>food</strong>s to their micr<strong>on</strong>utrient status.Fulfilment of these objectives not <strong>on</strong>ly requires political commitment and corporatesupport, but also that nati<strong>on</strong>al laws and regulati<strong>on</strong>s, manufacturingand marketing practices, and community norms, policies and structures bestrengthened or modified in some way so as to bring adequately fortified<strong>food</strong>s to those who need them most. Furthermore, individuals are likely to needguidance and encouragement before they willingly incorporate fortified productsinto their diets, modify their dietary practices that affect the absorpti<strong>on</strong> ofnutrients in <strong>food</strong>s, and adopt household storage and cooking techniques thatmaximize the nutrient value of the <strong>food</strong>s they eat. Throughout the entirety ofthis individual behaviour–envir<strong>on</strong>ment change c<strong>on</strong>tinuum, communicati<strong>on</strong> playsa critical role.To increase its chances of success, a fortificati<strong>on</strong> programme needs to besupported by a range of well-coordinated communicati<strong>on</strong> activities that promoteindividual, community, corporate and political change. In this respect it isimportant to be aware that messages about the benefits of fortificati<strong>on</strong> can becommunicated in a number of different ways, using a variety of techniques, tovery different effect depending <strong>on</strong> the intended audience. By outlining someof the opti<strong>on</strong>s available, the main purpose of this chapter is, therefore, tohelp micr<strong>on</strong>utrient programme managers understand the different communicati<strong>on</strong>needs of various sectors and so direct their communicati<strong>on</strong> activities moreefficiently.224
10. COMMUNICATION, SOCIAL MARKETING, & ADVOCACY10.1 Communicati<strong>on</strong> strategies: the opti<strong>on</strong>sThere are a number of recognized methodologies that are available to programmemanagers for communicating messages about the benefits of micr<strong>on</strong>utrientfortificati<strong>on</strong>; these include nutriti<strong>on</strong> educati<strong>on</strong>, social marketing andadvocacy (see Table 10.1). Experience has shown some approaches to be particularlyuseful for encouraging individuals to adopt healthier behaviours (e.g.health communicati<strong>on</strong>, nutriti<strong>on</strong> educati<strong>on</strong>, social marketing); others havehelped foster community support, led to the introducti<strong>on</strong> of laws or regulati<strong>on</strong>sor mobilized entire countries for periodic health acti<strong>on</strong>s (e.g. advocacy, socialmobilizati<strong>on</strong>). In practice, however, it is not simply a questi<strong>on</strong> of choosing <strong>on</strong>eapproach over another, but finding the right blend of strategies and tactics thattogether achieve programme objectives (367).A useful framework for analysing communicati<strong>on</strong> needs, in which educati<strong>on</strong>,marketing and legislati<strong>on</strong> are viewed as interc<strong>on</strong>nected approaches to managingsocial and health issues, has been suggested by Rothschild (373). By describingthe relati<strong>on</strong>ship between various activities in terms of individual decisi<strong>on</strong>-makingand perceived costs and benefits (Figure 10.1), Rothschild’s framework canassist in identifying which approaches are best suited to which tasks.TABLE 10.1Nutriti<strong>on</strong> promoti<strong>on</strong> methods definedC<strong>on</strong>ceptNutriti<strong>on</strong>educati<strong>on</strong>Healthcommunicati<strong>on</strong>SocialmarketingAdvocacySocialmobilizati<strong>on</strong>Definiti<strong>on</strong>Any set of learning experiences designed to facilitate the voluntaryadopti<strong>on</strong> of eating and other nutriti<strong>on</strong>-related behaviours c<strong>on</strong>ducive tohealth and well-being (368).The crafting and delivery of messages and strategies, based <strong>on</strong>c<strong>on</strong>sumer research, to promote the health of individuals andcommunities (V. Freimuth in (369).“The design, implementati<strong>on</strong>, and c<strong>on</strong>trol of programmes aimed atincreasing the acceptability of a social idea, practice [or product] in <strong>on</strong>eor more groups of target adopters. The process actively involves thetarget populati<strong>on</strong>, who voluntarily exchange their time and attenti<strong>on</strong> forhelp in meeting their health needs as they perceive them” (370).Persuading others to support an issue of c<strong>on</strong>cern to an individual,group or community. May involve, “the strategic use of the mass mediaas a resource to advance a social or public policy initiative” (371).A broad scale movement to engage large numbers of people in acti<strong>on</strong>for achieving a specific development goal through self-reliant effort.Social mobilizati<strong>on</strong> is most effective when it is composed of a mix ofadvocacy, community participati<strong>on</strong>, partnerships and capacity-buildingactivities that together create an enabling envir<strong>on</strong>ment for sustainedacti<strong>on</strong> and behaviour change (372).225
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSFIGURE 10.1Relati<strong>on</strong>ship between individual decisi<strong>on</strong>-making and the perceived costs andbenefits of any new behaviour, idea or productPerceived benefits highPerceived costs lowEducati<strong>on</strong>MARKETINGPerceived costs highLaw/policyPerceived benefits lowSource: reproduced from reference (374), <strong>with</strong> the permissi<strong>on</strong> of the publishers.10.1.1 Educati<strong>on</strong>The upper left-hand quadrant of Figure 10.1 is occupied by educati<strong>on</strong> or “providingknowledge”. This approach is most effective when the benefits of achange are obvious, and the change does not appear costly to the pers<strong>on</strong> orgroup being asked to make the change. It had been assumed in the past that<strong>on</strong>ly a minimal amount of communicati<strong>on</strong> was needed to “educate” the public,opini<strong>on</strong> leaders in the scientific community and industry about the benefits ofadding nutrients to <strong>food</strong>s. However, experience <strong>with</strong> salt iodizati<strong>on</strong> has dem<strong>on</strong>stratedthat in reality a far more negotiated approach is required.Fortified products are developed to address a biological need for micr<strong>on</strong>utrients.However, at the individual level this need is largely unrecognized becausepeople neither crave micr<strong>on</strong>utrients nor realize that they are deficient. Instead,a populati<strong>on</strong>’s need for micr<strong>on</strong>utrients is defined by the health community,usually in terms of a biochemical, clinical or some other marker of deficiency.Since raw data <strong>on</strong> the prevalence of deficiency are often difficult for the generalpublic to understand, by themselves they do not suffice for providing individuals<strong>with</strong> a believable rati<strong>on</strong>ale for changing their shopping, <strong>food</strong> preparati<strong>on</strong> ordietary habits. What is needed instead is a more user-friendly message, preferably<strong>on</strong>e that is tailored to suit the informati<strong>on</strong> needs and cognitive ability of therecipients (see Box 10.1).Lack of ambiguity in educati<strong>on</strong>al messages is vital. Whenever technicalexperts disagree, the public tends to ignore all scientific evidence until such time226
10. COMMUNICATION, SOCIAL MARKETING, & ADVOCACYBOX 10.1Educati<strong>on</strong> as a communicati<strong>on</strong> strategy: keys to successEducati<strong>on</strong>al approaches work best when the recipient of the informati<strong>on</strong> hasalready expressed a desire or commitment to perform the desired behaviourand is now seeking informati<strong>on</strong> <strong>on</strong> what to do and how to do it.Informati<strong>on</strong> for the purpose of providing knowledge must be simple, clear andunambiguous. It should be:— adjusted to the cognitive abilities of the learner (i.e. according to age, educati<strong>on</strong>allevel, literacy and language of maximum understanding);— adjusted to the communicati<strong>on</strong> medium be it oral, visual, or tactile (e.g.mixing instructi<strong>on</strong>s);— answer factual questi<strong>on</strong>s such as What? Whom? Where? How?a unified message has emerged. For fortificati<strong>on</strong> programme managers it cansometimes be difficult to achieve a c<strong>on</strong>sensus between competing claims ofeffectiveness, safety, quality and cost of a given interventi<strong>on</strong>. For instance,whereas public health professi<strong>on</strong>als tend to advocate the most appropriate fortificantlevels for maximum impact, or recommend use of those fortificant compoundsthat offer the highest bioavailability, producers will try to minimizechanges in product quality and cost. A process which successfully negotiatesthese varying perspectives am<strong>on</strong>g public and private sectors is critical to developinga product profile that has the support of both government and industry– and that ultimately will be accepted by the c<strong>on</strong>sumer. Hence, at the outset of<strong>food</strong> fortificati<strong>on</strong> programmes, it is important to attempt to integrate and translatethe technical language and jarg<strong>on</strong> of the public health, <strong>food</strong> science andbusiness sectors into a comm<strong>on</strong> vocabulary that all the various professi<strong>on</strong>alsinvolved can understand. Technical language and jarg<strong>on</strong> should be reserved forprofessi<strong>on</strong>al communicati<strong>on</strong>s; the public will require a more carefully craftedapproach altogether, and <strong>on</strong>e that is based <strong>on</strong> the appearance of scientific c<strong>on</strong>sensusin order to achieve maximum penetrati<strong>on</strong>.10.1.2 Laws, policy and advocacy: communicating <strong>with</strong> policy-makersIn direct c<strong>on</strong>trast to educati<strong>on</strong>, and occupying the diag<strong>on</strong>ally opposite quadrantin Figure 10.1, laws (or regulati<strong>on</strong>s) are used to prompt societal change whena change appears to be costly and to compromise individual benefits. In thec<strong>on</strong>text of health, most laws and regulati<strong>on</strong>s are aimed at achieving the collectivegood over individual desires or profit. Rothschild defines law as “the useof coerci<strong>on</strong> to achieve behaviour in a n<strong>on</strong>-voluntary manner” (373), but in227
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSBOX 10.2Advocacy as a tool for communicating <strong>with</strong> policy-makers:keys to successAdvocacy frames issues for public attenti<strong>on</strong>, the media and policy-makers. Formaximum impact, advocacy should be:— focused <strong>on</strong> <strong>on</strong>e or a very limited number of issues;— get to the point quickly and end quickly;— add emoti<strong>on</strong>al c<strong>on</strong>tent and localizati<strong>on</strong>;— answer the questi<strong>on</strong>: Why should we care?practice laws can really <strong>on</strong>ly persuade, as individuals or entities can alwayschoose whether or not to obey a law or regulati<strong>on</strong> according to their owncost–benefit calculati<strong>on</strong>.When any c<strong>on</strong>cerned group organizes to change a law or policy, their primarytool is advocacy.Thus the recipient of advocacy is an individual or group <strong>with</strong>the power to change the law or regulati<strong>on</strong>, i.e. policy-makers. The primarymessage that needs to be communicated is why this individual or group shouldcare (Box 10.2). “Ideally, policy-makers should incorporate scientific informati<strong>on</strong>when ...making decisi<strong>on</strong>s. In reality, many decisi<strong>on</strong>s are based <strong>on</strong> shorttermdemands rather than l<strong>on</strong>g-term study, and policies and programmes arefrequently developed around anecdotal evidence. Existing health data are oftenunder-utilized and sometimes ignored” (375).Some advocacy groups (and indeed governments and corporati<strong>on</strong>s) activelycourt the mass media to draw attenti<strong>on</strong> to a particular issue and to present theirstandpoint. Micr<strong>on</strong>utrient programme managers wishing to use the media inthis way are advised to develop good working relati<strong>on</strong>ships <strong>with</strong> key journalistsand thus earn a reputati<strong>on</strong> for being a reliable source of informati<strong>on</strong>. It is helpfulto develop fact sheets, press briefings and other background materials that canbe used by the news media, but in order to maximize their impact, these usuallyneed to be combined <strong>with</strong> a “newsworthy” event, such as the release of newdata, a public meeting or a decisi<strong>on</strong> taken by the government. Although mediasuccess can be measured by the volume of exposure or “airtime”, ensuring thata story is presented the way it was intended is equally, if not more, important.This can be extremely difficult as the news media is increasingly an “entertainmentindustry,” and what drives story placement is often linked to what bringshigh levels of audience attenti<strong>on</strong>. Strategies for increasing media coverage arepresented in Box 10.3.228
10. COMMUNICATION, SOCIAL MARKETING, & ADVOCACYBOX 10.3Using the media: keys to successChances of obtaining media coverage are enhanced by:— c<strong>on</strong>flict, c<strong>on</strong>troversy or injustice;— community involvement;— ir<strong>on</strong>y;— a hard news “link” (timeliness);— images, i.e. pictures, video films, photographs, graphs, and expert interviews.10.1.3 Social marketingThe central part of Figure 10.1 is occupied by marketing, or, more specifically,by social marketing. Social marketing is the use of marketing techniques developedby the private sector (i.e. commercial marketing) to achieve public sectorgoals. In the area of public health, this technique has been used successfully tosupport family planning, HIV preventi<strong>on</strong>, oral rehydrati<strong>on</strong>, hand washing, andimmunizati<strong>on</strong>, as well as various nutriti<strong>on</strong> programmes, such as infant feeding,salt iodizati<strong>on</strong>, ir<strong>on</strong> supplementati<strong>on</strong> and dietary diversificati<strong>on</strong> programmes(376,377)Commercial and social marketing are alike in that both attempt to influenceindividuals to make choices c<strong>on</strong>cerning their behaviour, and/or the productsor services (the “offering”) they use by increasing the perceived value of theoffering and mitigating the perceived obstacles to its use. In commercialmarketing, c<strong>on</strong>sumers buy a product or service that they judge to be a fairtrade for the sum of m<strong>on</strong>ey paid. The profits generated through this exchangeare distributed back to the company that produced the product or service andits stockholders.The term “social marketing” is generally used to describe the promoti<strong>on</strong> ofthose “causes judged by pers<strong>on</strong>s in positi<strong>on</strong>s of power and authority to be beneficialto both individuals and society” (378). The potential c<strong>on</strong>sumer in a socialmarketing programme might be asked to use a product (e.g. a polio vaccine, avitamin A capsule, soap), a service (e.g. well-baby visit, preventive dental checkup)or adopt or modify a behaviour (e.g. mix an oral rehydrati<strong>on</strong> soluti<strong>on</strong>, refusean offer of a cigarette, breastfeed exclusively for 6 m<strong>on</strong>ths). Usually, the potentialc<strong>on</strong>sumer or “adopter” initially feels no need or desire for this product,service or modified behaviour, and, in fact, uses or does something else instead.229
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSWhen the exchange is completed, the c<strong>on</strong>sumer or adopter will have given uptime, a previously held belief or attitude, m<strong>on</strong>ey, or even all three to acquire theoffering. In a social marketing programme, unlike the commercial marketing scenario,the return to the “stockholders” is better health and welfare for the society.However, when social and private sector entities combine to market socially beneficialproducts, such as fortified <strong>food</strong>s, a reas<strong>on</strong>able m<strong>on</strong>etary profit is usuallyalso generated. This means that the venture can be made self-sustaining,and thus avoid having to rely <strong>on</strong> c<strong>on</strong>stant inputs from governments or d<strong>on</strong>oragencies (379,380).As the objective is largely a voluntary exchange, social marketing worksbest when it involves potential c<strong>on</strong>sumers in every aspect of a programme.Potential c<strong>on</strong>sumers need to be c<strong>on</strong>sulted about product or service developmentas well as its cost (or “price”), image (“product positi<strong>on</strong>ing”), distributi<strong>on</strong>(or “place”) and promoti<strong>on</strong>. These factors are referred to as the four“Ps” of social marketing, and are analysed in the c<strong>on</strong>text of <strong>food</strong> fortificati<strong>on</strong> inBox 10.4.Social marketing programmes require c<strong>on</strong>siderable investment in order tocreate awareness of the offerings and to dem<strong>on</strong>strate their value to potentialadopters to the extent that adopters are willing to exchange their time, m<strong>on</strong>eyor dearly held beliefs or habits for them. Social marketing programmes relyheavily <strong>on</strong> communicati<strong>on</strong>, and need time to develop to their full potential.However, social marketing is not just about communicati<strong>on</strong>; no amount of advertising,or advocacy, social mobilizati<strong>on</strong>, educati<strong>on</strong>, informati<strong>on</strong> or health communicati<strong>on</strong>can sell an inferior product that is badly packaged and distributedand/or unfavourably priced. For these reas<strong>on</strong>s, social marketing objectivesshould be defined, al<strong>on</strong>gside other programme objectives, at the planning stageof a fortificati<strong>on</strong> programme. Social marketing indicators should also be developedat this time; these can used, in combinati<strong>on</strong> <strong>with</strong> other programme indicators,to evaluate programme implementati<strong>on</strong> and performance (see Chapter9). C<strong>on</strong>sumer behaviour and its antecedents can be useful complementarymeasures of programme success.10.2 Communicati<strong>on</strong> to support social marketing programmesAs indicated in the previous secti<strong>on</strong>, the right mix of social marketing and otherstrategies can make all the difference to the success of a public health programme.Food fortificati<strong>on</strong> programme managers can make use of any <strong>on</strong>e ofa number of published resources to guide the development of the communicati<strong>on</strong>comp<strong>on</strong>ent of a social marketing programme to support their ownfortificati<strong>on</strong> initiative. CDCynergy. A communicati<strong>on</strong>s guide for micr<strong>on</strong>utrientinterventi<strong>on</strong>s is <strong>on</strong>e such resource that not <strong>on</strong>ly provides step-by-step guidancein such matters, but draws <strong>on</strong> examples of successfully marketed micr<strong>on</strong>utrient230
10. COMMUNICATION, SOCIAL MARKETING, & ADVOCACYBOX 10.4Keys to success in social marketing: the four “Ps” for fort i fie d<strong>food</strong>sProduct positi<strong>on</strong>ing■ A high quality fortified product should be produced in accordance <strong>with</strong> goodmanufacturing practice, WHO technical guidelines or some other form ofguidelines and regulati<strong>on</strong>s.■ Product presentati<strong>on</strong> should be attractive, tasty and in all ways appealing tothe potential c<strong>on</strong>sumer.■ Positi<strong>on</strong>ing of the product is derived through research <strong>with</strong> the potential c<strong>on</strong>sumer.It makes a promise that can be kept. Eventually this will become a“brand”.Price■ The fortified product should be packaged in quantities and priced so as tobe affordable to the potential c<strong>on</strong>sumer.■ Different quantities/price points might be developed to satisfy differentgroups of c<strong>on</strong>sumers.Place■ The fortified product needs to be widely distributed (including rural areas)using commercial <strong>food</strong> distributi<strong>on</strong> channels, where appropriate.■ All physical barriers to obtaining the fortified product should be eliminated.Promoti<strong>on</strong>■ Product promoti<strong>on</strong> should be driven by the product’s positi<strong>on</strong>ing.■ The benefits of fortified <strong>food</strong>s and the limitati<strong>on</strong>s of n<strong>on</strong>-fortified substitutesneed to be presented in terms that are meaningful to the c<strong>on</strong>sumer.■ The act of purchasing fortified <strong>food</strong>s needs to be presented as “new”, andthen eventually, as “normal”.■ The c<strong>on</strong>sumer should be persuaded to adopt c<strong>on</strong>sumpti<strong>on</strong> practices thatenhance the absorpti<strong>on</strong> of micr<strong>on</strong>utrients.■ The c<strong>on</strong>sumer should be educated to store fortified <strong>food</strong>s in ways that protectthe product and prol<strong>on</strong>g its shelf-life.231
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSprogrammes from around the world 1 . In this particular guide, all promoti<strong>on</strong>almaterials producti<strong>on</strong> and messaging is based <strong>on</strong> a systematic, data-drivenprocess that is centred <strong>on</strong> the intended audience or the c<strong>on</strong>sumer. This processrequires the following activities:• Qualitative and quantitative research to define participating audiences, c<strong>on</strong>sumerattitudes and barriers to change.• Data analysis to define and segment audiences into like groups forcommunicati<strong>on</strong>.• Research and pre-testing to determine the most motivating benefits for thesetarget audiences.• Message creati<strong>on</strong> based <strong>on</strong> key benefits. For each segment, messages mustanswer the questi<strong>on</strong>, “What is the benefit to me?” Background and qualitativeresearch can define the key messages that answer that questi<strong>on</strong>.• Promoti<strong>on</strong>s and other activities that are disseminated via channels appropriateto each audience segment.This approach can be applied to other communicati<strong>on</strong> strategies, includingadvocacy and nutriti<strong>on</strong> educati<strong>on</strong>, to make them more tailored and effective.Social marketing research methods can also be used to interact <strong>with</strong> all participantsin a micr<strong>on</strong>utrient programme, i.e. not just potential c<strong>on</strong>sumers, but alsoindustry representatives, and government and n<strong>on</strong>governmental organizati<strong>on</strong>s(NGOs).The guidance and suggesti<strong>on</strong>s for communicating the benefits of fortificati<strong>on</strong>that is provided in this part of the <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> is necessarily generic. It is recommendedthat social marketing research be c<strong>on</strong>ducted in each country orregi<strong>on</strong> in order to identify the right mix of messages and communicati<strong>on</strong>s thatare going to support fortificati<strong>on</strong> programme goals to best effect.10.2.1 Building collaborative partnershipsIn some parts of the world, the formati<strong>on</strong> of alliances or networks has led to amore effective collaborati<strong>on</strong> between the main partners involved in the c<strong>on</strong>trolof MNM.These partnerships typically include representatives from bilateral andmultinati<strong>on</strong>al agencies, internati<strong>on</strong>al and nati<strong>on</strong>al NGOs, research and academicinstituti<strong>on</strong>s, foundati<strong>on</strong>s and increasingly, industry. The Network for Sustained1CDCynergy. A communicati<strong>on</strong>s guide for micr<strong>on</strong>utrient interventi<strong>on</strong>s is a comprehensive CD-ROMthat helps plan, implement and evaluate communicati<strong>on</strong>s programmes. The CD-ROM is available,free of charge, from the Centres for Disease C<strong>on</strong>trol and Preventi<strong>on</strong> and can be ordered<strong>on</strong>line via: http://www.cdc.gov/nccdphp/dnpa/immpact/tools/order_form.htm.232
10. COMMUNICATION, SOCIAL MARKETING, & ADVOCACYEliminati<strong>on</strong> of Iodine Deficiency 1 and the Global Alliance for Improved Nutriti<strong>on</strong>(GAIN) 2 and the Flour Fortifcati<strong>on</strong> Initiative 3 are am<strong>on</strong>g the better-knownexamples. The primary role of these alliances has been to mobilize decisi<strong>on</strong>makers<strong>with</strong> regard to the public health dimensi<strong>on</strong> of MNM and to providesupport for <strong>food</strong> fortificati<strong>on</strong> programmes.Within this atmosphere of multisectoral collaborati<strong>on</strong>, the primary role of the<strong>food</strong> industry is to produce, distribute and market a good quality, competitivelypricedfortified product. Ideally, fortificati<strong>on</strong> should add no more than a few percent of product cost to the product price, have no detrimental impact <strong>on</strong> productquality and not create imbalances in the business or competitive envir<strong>on</strong>ment.The public sector, <strong>on</strong> the other hand, is resp<strong>on</strong>sible for creating an envir<strong>on</strong>mentthat allows the private sector to invest in fortificati<strong>on</strong>.This enabling envir<strong>on</strong>mentshould minimize unfair competiti<strong>on</strong> from lower quality or cheaper unfortified<strong>food</strong>s that make it difficult to pass added costs of fortificati<strong>on</strong> <strong>on</strong> to thec<strong>on</strong>sumer.Inevitably there will be some tensi<strong>on</strong> between the public sector emphasis <strong>on</strong>c<strong>on</strong>sumer rights and <strong>on</strong> equity and health issues, and the private sector focus<strong>on</strong> c<strong>on</strong>sumer demand, commercial viability and revenue generati<strong>on</strong>. Balancingthe public and private perspectives requires developing communicati<strong>on</strong> channelsfor negotiating a number of potentially c<strong>on</strong>tentious issues, such as:• Increasing sales is a fundamental goal of private sector marketing. However,this is not necessarily a public or nati<strong>on</strong>al goal, and for some <strong>food</strong> vehicles,such as sugar or salt, increased c<strong>on</strong>sumpti<strong>on</strong> – or sales – is not an explicitgoal of the programme. Messages should lead c<strong>on</strong>sumers to the fortifiedproduct, but not necessarily to increase overall c<strong>on</strong>sumpti<strong>on</strong> of thecommodity (i.e. sugar, oil, salt, flour).• Whereas private companies strive to maximize revenues, the public sectorstrives to maximize accessibility and minimize any price rise. A balance thusneeds to be struck whereby producers are fairly compensated, while avoidingany sharp price increases for at-risk c<strong>on</strong>sumers.• Logos and endorsements awarded by governments or NGOs can be powerfulpromoti<strong>on</strong>al tools. The difficulty here lies in the fact that, while the privatesector promoti<strong>on</strong>s are designed to maximize market share, public campaignscannot be seen to be favouring specific companies. One possible soluti<strong>on</strong> isto use public promoti<strong>on</strong>s that are generic to fortificati<strong>on</strong>, to the micr<strong>on</strong>utrientsc<strong>on</strong>cerned, to the seal of recogniti<strong>on</strong> or to the <strong>food</strong> vehicle.1Further informati<strong>on</strong> is available via the Internet at: http://www.sph.emory.edu/iodinenetwork/.2Further informati<strong>on</strong> is available via the Internet at: http://www.gainhealth.org.3Further informati<strong>on</strong> is available via the Internet at: http://www.sph.emory.edu/wheatflour/.233
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSBOX 10.5Engendering Collaborative Partnerships: The role of amultisectoral fort i ficati<strong>on</strong> task force or committeeAlthough informal work groups can provide channels for communicati<strong>on</strong>, a moreformal body, where members officially represent the interests of their organizati<strong>on</strong>s,can be more effective in opening communicati<strong>on</strong> channels in the rightway al<strong>on</strong>g the chain – from high-level decisi<strong>on</strong>-makers through to c<strong>on</strong>sumers.A multisectoral task force or committee is useful for securing commitment,gaining c<strong>on</strong>sensus and for coordinating the c<strong>on</strong>tributi<strong>on</strong>s of various sectors ordisciplines (380). Participati<strong>on</strong> should include stakeholders involved in the technicalimplementati<strong>on</strong> of fortificati<strong>on</strong>, as well as those offering credible channelsto key audiences, instituti<strong>on</strong>s and decisi<strong>on</strong>-makers. While participants will varyaccording to nati<strong>on</strong>al circumstances, the core group might include:■ government health, regulatory and <strong>food</strong> c<strong>on</strong>trol agencies involved in regulati<strong>on</strong>,m<strong>on</strong>itoring and surveillance, as well as agencies that deal <strong>with</strong> specialfinancing needs;■ companies involved in the producti<strong>on</strong> of the choosen <strong>food</strong> vehicle, valueaddedprocessing, and the wholesale and retail distributi<strong>on</strong> of the fortifiedproduct;■ academic and research instituti<strong>on</strong>s (which provide technical input as well ascredibility);■ NGOs (which offer support, resources and channels of communicati<strong>on</strong> to arange of c<strong>on</strong>stituencies).■ C<strong>on</strong>sumer organizati<strong>on</strong>s.In some settings, the best way to make sure that lines of communicati<strong>on</strong>between programme partners are open may be to establish a formal multisectoraltask force or committee. The role and membership of such a body arediscussed further in Box 10.5.10.2.2 Developing messages for government leadersFor many nati<strong>on</strong>al governments, fortificati<strong>on</strong> is an attractive opti<strong>on</strong> because itoffers an opportunity to achieve nati<strong>on</strong>al health and nutriti<strong>on</strong> goals that – byshifting the costs to the market – can be substantially financed by the privatesector. Naturally, government departments and agencies vary in their prioritiesand so some will have a greater interest in certain outcomes than others. Forinstance, the potential for improved productivity and nati<strong>on</strong>al ec<strong>on</strong>omic developmentwill be of particular interest to departments resp<strong>on</strong>sible for finance andrevenue. Messages framed by nati<strong>on</strong>al ec<strong>on</strong>omic circumstances are thus more234
10. COMMUNICATION, SOCIAL MARKETING, & ADVOCACYlikely to strike a chord <strong>with</strong> policy-makers working in these areas 1 . Other examplesinclude:• Messages defining reduced health care costs will have particular res<strong>on</strong>anceam<strong>on</strong>g officials resp<strong>on</strong>sible for health budgets 2 .• Outcomes such as improved cognitive ability and improved school performancecan be persuasive for agencies investing in educati<strong>on</strong>al programmes.• Agencies dealing <strong>with</strong> industrial development or public works are most likelyto be motivated by estimates of depressed productivity and ec<strong>on</strong>omic loss.Depending <strong>on</strong> nati<strong>on</strong>al circumstances, some government departments may havevery specific c<strong>on</strong>cerns about the impact of fortificati<strong>on</strong> and thus may be primetargets for advocacy. Having identified, through social marketing research, thespecific interests and c<strong>on</strong>cerns of each group, advocacy or educati<strong>on</strong> sessi<strong>on</strong>scan then be tailored to address these. For instance:• In some countries, government agencies are involved in the producti<strong>on</strong>, distributi<strong>on</strong>or subsidy of staple <strong>food</strong>s. Fortificati<strong>on</strong> will impact the budget ofministries or agencies resp<strong>on</strong>sible for the financing of these activities.• Sometimes the selected fortificati<strong>on</strong> vehicle (often wheat flour) is imported,in which case, officials may have specific c<strong>on</strong>cerns about promoting a productthat has a negative impact <strong>on</strong> the nati<strong>on</strong>al balance sheet.• Since fortificati<strong>on</strong> is more cost-effective when adopted by larger or moremodern industries, agencies resp<strong>on</strong>sible for small business development maybe c<strong>on</strong>cerned about the social and ec<strong>on</strong>omic impact <strong>on</strong> small producers, theirfamilies and communities.• Creating an enabling envir<strong>on</strong>ment for private investment often involves providingexempti<strong>on</strong>s from specific taxes and duties. The ministries resp<strong>on</strong>siblefor administering these revenue-creating programmes are often deluged <strong>with</strong>exempti<strong>on</strong> requests.10.2.3 Developing messages for industry leadersFrom the point of view of private producers, fortificati<strong>on</strong> cannot be allowed t<strong>on</strong>egatively impact fundamental business goals, i.e. sales and profits. Any new1A number of tools are available for estimating the ec<strong>on</strong>omic impact of micr<strong>on</strong>utrient deficienciesbased <strong>on</strong> nati<strong>on</strong>al statistics for prevalence, gross domestic product, workforce structure, healthcare utilizati<strong>on</strong> and other country-specific factors. Profiles, a computer simulati<strong>on</strong> developed bythe Academy for Educati<strong>on</strong> Development, Washingt<strong>on</strong>, DC, USA is <strong>on</strong>e such tool. More informati<strong>on</strong>is available through the internet at http://www.aedprofiles.org/. Another is that developedby the Micr<strong>on</strong>utrient Initiative, Ottawa, Canada, details of which are given in the report titled,Ec<strong>on</strong>omic c<strong>on</strong>sequences of ir<strong>on</strong> deficiency (365).2The Profiles simulati<strong>on</strong> (see above) includes a methodology for measuring the reducti<strong>on</strong> in healthexpenditures.235
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSproduct launch carries <strong>with</strong> it the risk of c<strong>on</strong>sumer resistance and, therefore, aloss of sales and reduced profits. Messages for industry can address this c<strong>on</strong>cernby highlighting past successful commercial experiences or <strong>on</strong>going trials thatindicate little or no c<strong>on</strong>sumer resistance to fortified products.Although from the c<strong>on</strong>sumer perspective, fortificati<strong>on</strong> usually entails <strong>on</strong>ly avery small annual cost, for a large producer, it can mean a large initial investment.To help overcome any reticence <strong>on</strong> the part of industry to make the necessaryinvestment, messages directed at the industry sector need to stress publicsector commitment to creating an “enabling market envir<strong>on</strong>ment”, that is to say,<strong>on</strong>e that allows business to make a reas<strong>on</strong>able profit or at the very least recouptheir investment. While this involves a number of technical, commercial and regulatoryfactors, a key element is the creati<strong>on</strong> of c<strong>on</strong>sumer awareness anddemand. Therefore, messages to industry should also highlight public sectorcommitment to developing communicati<strong>on</strong> channels and to providing supportfor credible health claims and public endorsements, such as official logos.Bey<strong>on</strong>d the basic messages about enabling c<strong>on</strong>diti<strong>on</strong>s of sales and profits, anumber of other messages may be useful for securing industry commitment tofortificati<strong>on</strong> programmes. Again, depending <strong>on</strong> the results of research involvingindustry and government sector representatives, messages that express the followingideas may be helpful:• In the eyes of public or government relati<strong>on</strong>s departments, the promise of animproved public image and better government relati<strong>on</strong>s is often perceived asa benefit to business.• For technical audiences, fortificati<strong>on</strong> could be presented as an opportunity toimprove product quality. For example, in the case of flour millers, addingmicr<strong>on</strong>utrients can be framed as restoring milled flour to the original nutriti<strong>on</strong>alquality of the whole grain.• For producti<strong>on</strong> managers in developing countries, reference to fortificati<strong>on</strong>in North America and Europe can suggest industry best practice.• For some companies, expanded market share and c<strong>on</strong>sumer brand loyaltymay be perceived as a potential business benefit of fortificati<strong>on</strong>. However,although some companies may benefit more than others, there is little evidencethat nati<strong>on</strong>al fortificati<strong>on</strong> increases sales overall.Nor should the power of the argument that fortificati<strong>on</strong> is “Doing the rightthing” be underestimated. While largely focused <strong>on</strong> the bottom line, industrydoes have a social c<strong>on</strong>science. Moreover, industry is very c<strong>on</strong>cerned about c<strong>on</strong>sumerawareness and the reacti<strong>on</strong> to a new product. This interest is not c<strong>on</strong>finedto the industry sector; policy-makers and business leaders are also sensitiveto potential c<strong>on</strong>sumer reacti<strong>on</strong>. For government leaders, c<strong>on</strong>sumers are alsopolitical c<strong>on</strong>stituents, and they too need to anticipate potential public reacti<strong>on</strong>236
10. COMMUNICATION, SOCIAL MARKETING, & ADVOCACYto fortificati<strong>on</strong>. Therefore, even though they may not be a direct audience foradvocacy, understanding the c<strong>on</strong>sumer is critical to answering the c<strong>on</strong>cerns ofleadership audiences and for developing effective messages.10.2.4 Developing c<strong>on</strong>sumer marketing strategies and educati<strong>on</strong>The goal of c<strong>on</strong>sumer marketing and educati<strong>on</strong> is to create a percepti<strong>on</strong> of valuein fortificati<strong>on</strong>, so that c<strong>on</strong>sumers will accept the new product, choose fortifiedover n<strong>on</strong>-fortified products, and if necessary, pay a slightly higher price. Creatingc<strong>on</strong>sumer demand for fortified <strong>food</strong>s, particularly am<strong>on</strong>g lower incomec<strong>on</strong>sumers, can encounter steep barriers, especially in a highly-competitiveenvir<strong>on</strong>ment (see Box 10.6).BOX 10.6F o rt i fied products and c<strong>on</strong>sumer barr i e r sPossible c<strong>on</strong>sumer barriers to fortified <strong>food</strong>s and products include thefollowing:■ Research in many countries indicates that nutriti<strong>on</strong>al benefits, while an importantfeature, is a low purchase priority. Price, taste, packaging, accessibilityand c<strong>on</strong>venience are almost invariably of higher priority.■ The need for micr<strong>on</strong>utrients is often unrecognized by the c<strong>on</strong>sumer, and mustbe made visible. This is a difficult task.■ The benefits of fortificati<strong>on</strong> are subtle. Because fortified <strong>food</strong>s offer a preventiverather than a therapeutic benefit, no immediate satisfacti<strong>on</strong> is felt.Moreover, benefits such as improved school performance and greater productivityaccrue <strong>on</strong>ly years into the future. Promoting preventi<strong>on</strong> and futurebenefits is often particularly challenging.■ While the incremental price increase associated <strong>with</strong> fortificati<strong>on</strong> is nearly invisible,low-income c<strong>on</strong>sumers are keenly sensitive to any price differential. C<strong>on</strong>sumers,particularly the poor who tend to be at the greatest risk of MNM, arealso the most likely to purchase cheaper products or to seek alternatives.■ Staple <strong>food</strong>s are often seen as pure or natural products. C<strong>on</strong>sumer resistancemay emerge from misinformati<strong>on</strong> about adding “foreign” substancesor “additives.” These range from apprehensi<strong>on</strong>s about toxicity or changes inthe sensory qualities of a <strong>food</strong>, to fears about the true goal of the fortificati<strong>on</strong>programme.■ Staple <strong>food</strong>s and c<strong>on</strong>diments are part of cultural identity and c<strong>on</strong>sumers maysimply resist giving up the old for the new.■ In some cases, there is resistance from sometimes more affluent market segmentswho feel that they do not need additi<strong>on</strong>al micr<strong>on</strong>utrients, and believethey are being forced to purchase and c<strong>on</strong>sume fortified products.237
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSC<strong>on</strong>sumer marketing strategies can be divided into two categories, “push” and“pull”. A push or supply-driven strategy pre-empts the choice between a fortifiedand unfortified product by universal, and usually mandatory, regulati<strong>on</strong>. Intheory, while prices may rise as a result of the introducti<strong>on</strong> of mandatory fortificati<strong>on</strong>,there will be no price difference between competitive products as aresult of fortificati<strong>on</strong>. With little c<strong>on</strong>sumer choice or price competiti<strong>on</strong>, the c<strong>on</strong>sumerplays a less active role and thus communicati<strong>on</strong> strategies need to focus<strong>on</strong> ensuring c<strong>on</strong>sumer acceptance, awareness and educati<strong>on</strong>.When fortified <strong>food</strong>s compete <strong>with</strong> less expensive n<strong>on</strong>-fortified products inthe market-place, a demand-driven or pull strategy is needed. In this scenario, apercepti<strong>on</strong> of value must be created to compensate for the price difference andthe fortified product must be positively differentiated from the competiti<strong>on</strong> inorder to develop an active c<strong>on</strong>sumer preference. Communicati<strong>on</strong> strategiesfocused <strong>on</strong> generic c<strong>on</strong>sumer awareness and understanding may not always besufficient and sometimes more aggressive commercial marketing techniques arerequired in order to provide a competitive edge for fortified products.A collaborative alliance of government, industry and NGO representatives,al<strong>on</strong>g the lines previously described (see secti<strong>on</strong> 10.2.1) offers opportunitiesto reach c<strong>on</strong>sumers through a broad mix of public and private sector communicati<strong>on</strong>channels. These range from government broadcast televisi<strong>on</strong> and radio,through health or outreach centres, to various points of sale. Each of thesesectors also brings their own distinct brand of experience and expertise: publicsector agencies and many NGOs have l<strong>on</strong>g experience in health and nutriti<strong>on</strong>communicati<strong>on</strong>s, and in public educati<strong>on</strong> activities to raise health awareness andpromote healthy behaviours. The private <strong>food</strong> sector provides expertise in c<strong>on</strong>sumermarketing, <strong>with</strong> which to create product demand and c<strong>on</strong>sumer purchasingpreference. Opening multisectoral channels of communicati<strong>on</strong> andcooperati<strong>on</strong> is central to capitalizing <strong>on</strong> the unique strengths of each sector.10.3 Sustaining the programmeEven after fortified products have been launched and established in the marketplace, c<strong>on</strong>sumer and professi<strong>on</strong>al awareness remains critical to sustaining fortificati<strong>on</strong>programmes. Maintaining c<strong>on</strong>sumer support ensures that whengovernments change, fortificati<strong>on</strong>-friendly policies are sustained. Likewise,c<strong>on</strong>sumer awareness will help to secure stable and c<strong>on</strong>tinued industry supportdespite changes in market c<strong>on</strong>diti<strong>on</strong>s which may tempt companies to <strong>with</strong>drawfrom the programme or not comply <strong>with</strong> regulati<strong>on</strong>s, despite initially supportingfortificati<strong>on</strong>.C<strong>on</strong>tinued collaborati<strong>on</strong> between organizati<strong>on</strong>s and agencies involved in communicati<strong>on</strong>sand quality assurance is also vital to sustaining interest in fortificati<strong>on</strong>.For example, when awarding public sector symbols – logos, seals of238
10. COMMUNICATION, SOCIAL MARKETING, & ADVOCACYapproval or other forms of endorsement – product quality must be regularlyassured. The added value of the public symbol is <strong>on</strong>ly as good as the products<strong>with</strong> which it is associated and ultimately it is the credibility of the organizati<strong>on</strong>(s)standing behind the endorsement that is at stake. In countries where government<strong>food</strong> c<strong>on</strong>trol and enforcement is not fully functi<strong>on</strong>al, c<strong>on</strong>sumerorganizati<strong>on</strong>s offer a means by which to m<strong>on</strong>itor the marketplace. Under suchcircumstances, empowering c<strong>on</strong>sumer organizati<strong>on</strong>s to work <strong>with</strong> governmentand industry – by collecting samples or publishing results of product analysis –can be an important strategy to ensure quality assurance and evaluati<strong>on</strong>.SummaryThe chances of success of a fortificati<strong>on</strong> programme are greatly improved if it is supportedby a range of activities that collectively help to create an enabling envir<strong>on</strong>mentfor fortificati<strong>on</strong>. In practice this means promoting change at all levels – individual, community,corporate and political.Various ways of communicating messages about the benefits of fortificati<strong>on</strong> exist,including nutriti<strong>on</strong> educati<strong>on</strong>, social marketing and advocacy. Educati<strong>on</strong> strategieswork best when the benefits of change are obvious (the perceived benefits are high)and the change does not appear costly to the individual or group being asked to makethe change (i.e. perceived costs are low). C<strong>on</strong>versely, regulatory approaches may bemore appropriate when the perceived benefits of the change are low and the perceivedcosts are high. All fortificati<strong>on</strong> programmes will benefit from some form of socialmarketing, i.e. the use of commercial marketing techniques to achieve public sectorgoals. Social marketing is at its most effective when it involves the c<strong>on</strong>sumer in everyaspect of a programme, from product development to product positi<strong>on</strong>ing, placement,pricing and promoti<strong>on</strong>, and is based <strong>on</strong> qualitative and quantitative research that hasdefined the key c<strong>on</strong>sumer groups, their attitudes and barriers to change.Messages must be unambiguous and tailored to match the informati<strong>on</strong> needs andcognitive abilities of the recipient.Establishing some form of collaborative network or alliance can be a good way ofopening and maintaining communicati<strong>on</strong> channels am<strong>on</strong>g principal stakeholders. Thiscan also provide a forum for negotiating any c<strong>on</strong>flicts of interest that may arise betweenthe private and public sectors.239
CHAPTER 11Nati<strong>on</strong>al <strong>food</strong> lawGovernments have a key role to play in ensuring that <strong>food</strong> fortificati<strong>on</strong> is effectivefor the populati<strong>on</strong> group(s) most at risk from micr<strong>on</strong>utrient malnutriti<strong>on</strong>,but is safe for the populati<strong>on</strong> as a whole. Food laws and related measures,together <strong>with</strong> a broader <strong>food</strong> c<strong>on</strong>trol system, are the primary tools that are availableto governments for establishing an appropriate level of c<strong>on</strong>trol over <strong>food</strong>fortificati<strong>on</strong> practices.This chapter examines some of the technical and legal issues that are involvedin the development of nati<strong>on</strong>al <strong>food</strong> fortificati<strong>on</strong> law. The discussi<strong>on</strong> focuses <strong>on</strong>the regulati<strong>on</strong> of the compositi<strong>on</strong> of fortified <strong>food</strong>s and the labelling and advertisingof pre-packaged fortified <strong>food</strong> products. Other elements of nati<strong>on</strong>al <strong>food</strong>laws, for example those dealing <strong>with</strong> industry licensing, support or sancti<strong>on</strong>s arebey<strong>on</strong>d the scope of these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>. When establishing provisi<strong>on</strong> for fortificati<strong>on</strong><strong>with</strong>in nati<strong>on</strong>al <strong>food</strong> law, regulators will need to take account of existingregulati<strong>on</strong>s <strong>on</strong> internati<strong>on</strong>al trade and the global agreements that today increasinglygovern that trade. For this reas<strong>on</strong>, the chapter commences <strong>with</strong> a briefoverview of the internati<strong>on</strong>al systems for setting <strong>food</strong> standards and the currentglobal agreements <strong>on</strong> internati<strong>on</strong>al trade.11.1 The internati<strong>on</strong>al c<strong>on</strong>textTwo global trade agreements are relevant to <strong>food</strong>, both of which are administeredby the World Trade Organizati<strong>on</strong> (WTO). These are:• the Agreement <strong>on</strong> the Applicati<strong>on</strong> of Sanitary and Phytosanitary Measures(the SPS Agreement);• the Agreement <strong>on</strong> Technical Barriers to Trade (the TBT Agreement) (381).Food fortificati<strong>on</strong> measures, whether mandatory or voluntary, are most likely tobe covered by the latter, i.e. the TBT Agreement. This agreement recognizesthat:No country should be prevented from taking measures that are necessary forthe protecti<strong>on</strong> of human health at the levels it c<strong>on</strong>siders appropriate, subjectto the requirement that they are not applied in a manner which would c<strong>on</strong>-240
11. NATIONAL FOOD LAWstitute a means of arbitrary or unjustifiable discriminati<strong>on</strong> between countrieswhere the same c<strong>on</strong>diti<strong>on</strong>s prevail or a disguised restricti<strong>on</strong> <strong>on</strong> internati<strong>on</strong>altrade, and are otherwise in accordance <strong>with</strong> the provisi<strong>on</strong>s of this Agreement.In other words, countries may adopt provisi<strong>on</strong>s that restrict trade for legitimatereas<strong>on</strong>s – health being <strong>on</strong>e of them – providing such measures are in accordance<strong>with</strong> the five governing principles laid down in the TBT Agreement. These fivegoverning principles act to ensure that unnecessary obstacles to internati<strong>on</strong>altrade are not created. The key elements of the TBT Agreement relating to coverage,definiti<strong>on</strong>s, legitimate objectives and governing principles are explainedin Annex F.The TBT Agreement encourages the use of internati<strong>on</strong>al standards, exceptwhere they would be ineffective or inappropriate in the nati<strong>on</strong>al situati<strong>on</strong> (seewww.codexalimentarius.net and Annex F) (382).11.2 Nati<strong>on</strong>al <strong>food</strong> law and fortificati<strong>on</strong>Food law, operating in c<strong>on</strong>cert <strong>with</strong> the broader <strong>food</strong> c<strong>on</strong>trol system, is the mechanismcomm<strong>on</strong>ly used by governments to set technical provisi<strong>on</strong>s for fortified<strong>food</strong>s, the most important of which relate to their compositi<strong>on</strong>, labelling andclaims. (Claims are statements that manufacturers make in order to inform c<strong>on</strong>sumersabout their products.) Food law may also be used to impose broaderc<strong>on</strong>trols <strong>on</strong> the <strong>food</strong> industry, and to establish m<strong>on</strong>itoring and public informati<strong>on</strong>systems in support of <strong>food</strong> fortificati<strong>on</strong>.Food law typically has a number of objectives, the most important of whichis the protecti<strong>on</strong> of public health. Other frequently cited objectives are:— the provisi<strong>on</strong> of adequate informati<strong>on</strong> to enable informed choice;— the preventi<strong>on</strong> of fraud and misleading or deceptive c<strong>on</strong>duct;— fair trade.To meet these objectives, fortificati<strong>on</strong> provisi<strong>on</strong>s in <strong>food</strong> law should not <strong>on</strong>lyensure that all compositi<strong>on</strong>al parameters applicable to both fortificants and <strong>food</strong>vehicles deliver safe and appropriately efficacious public health outcomes butalso that the labelling, claims and advertising of fortified <strong>food</strong>s is factual andnot misleading, and provides sufficient informati<strong>on</strong> to enable appropriatec<strong>on</strong>sumpti<strong>on</strong>.11.2.1 Forms of <strong>food</strong> law: legislati<strong>on</strong>, regulati<strong>on</strong> andcomplementary measuresFood law comm<strong>on</strong>ly comprises proclaimed or decreed legislati<strong>on</strong> that establishesthe legal framework and the broad principles, accompanied by subordinate241
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTStechnical regulati<strong>on</strong>s that give detailed effect under or <strong>with</strong>in such legislati<strong>on</strong>.Thus <strong>food</strong> fortificati<strong>on</strong> requirements may be established either in an act of thegoverning legislature (such as a <strong>food</strong>- or health-related act), or in technical <strong>food</strong>regulati<strong>on</strong>s. An example of an act that is solely dedicated to mandatory fortificati<strong>on</strong>is the Philippines’ Act Promoting Salt Iodizati<strong>on</strong> Nati<strong>on</strong>wide (6). Thislaw establishes policy, applicability, industry support, public informati<strong>on</strong> andsancti<strong>on</strong>s, and is supported by rules and regulati<strong>on</strong>s for the implementati<strong>on</strong> ofsalt iodizati<strong>on</strong> and related purposes; these rules include a technical standard foriodized salt. Other countries use technical regulati<strong>on</strong>s (also called standards orother such similar terms), to mandate the specific legal requirements for <strong>food</strong>fortificati<strong>on</strong>, but rely <strong>on</strong> parent legislati<strong>on</strong> to ensure appropriate implementati<strong>on</strong>.One advantage of setting fortificati<strong>on</strong> provisi<strong>on</strong>s in regulati<strong>on</strong>, rather thanin legislati<strong>on</strong>, is that amendments can be made more quickly and easily, providingof course, that the power to administer regulati<strong>on</strong>s is delegated from theprimary governing legislature to an appropriate subsidiary or statutory body.Regardless of the way in which nati<strong>on</strong>al <strong>food</strong> law is c<strong>on</strong>structed, all thoseinvolved in the <strong>food</strong> producti<strong>on</strong> and distributi<strong>on</strong> system (including importers)must understand the applicable laws and, above all, comply <strong>with</strong> them. To thisend, and to ensure that <strong>food</strong> law achieves its public health objectives, it must be:— certain in its operati<strong>on</strong> (i.e. clearly and unambiguously expressed for thoseengaged in the activity to which the regulati<strong>on</strong> is directed);—supported by an appropriately structured and resourced informati<strong>on</strong> disseminati<strong>on</strong>system and enforcement capability.Under certain circumstances, complementary measures to government legislati<strong>on</strong>or regulati<strong>on</strong> can be used to fulfil regulatory objectives.These measures takethe form of industry self-regulati<strong>on</strong> or a co-regulatory mechanism betweenindustry and government in which government decides the appropriate level ofinvolvement. Such measures are respectively administered by industry al<strong>on</strong>e orjointly by industry and the government sector, and are best suited to matters ofprocess or intermediate outcome. A complementary system <strong>on</strong>ly works wellwhen the following recognized “success factors” are present:— the level of risk to public health and safety, or potential harm to c<strong>on</strong>sumers,is low;— the product is relatively homogeneous across the product category andc<strong>on</strong>sumers can readily identify it <strong>with</strong> the industry;— the industry is competitive, but also cohesive and represented by an activeindustry associati<strong>on</strong>;— the industry and/or its associati<strong>on</strong> is resp<strong>on</strong>sive to c<strong>on</strong>sumer complaints;242
11. NATIONAL FOOD LAW— companies are keen to enhance their future viability and are c<strong>on</strong>cernedabout their reputati<strong>on</strong>s, future customers and the wider community.11.2.2 Regulating <strong>food</strong> fortificati<strong>on</strong>: general c<strong>on</strong>siderati<strong>on</strong>sBefore deciding <strong>on</strong> the format and detail of fortificati<strong>on</strong> provisi<strong>on</strong>s, it is vital thatregulators understand the factors that shape their country’s <strong>food</strong> supply patterns.Important c<strong>on</strong>siderati<strong>on</strong>s might include the balance between domesticallyproducedand imported fortified products; the micr<strong>on</strong>utrient compositi<strong>on</strong> of theimported product; the capacity of the domestic industry to produce or increaseproducti<strong>on</strong> of the fortified product; and the industry’s overall cohesiveness.Having this understanding is especially pertinent if imported fortified productsare going to make a significant c<strong>on</strong>tributi<strong>on</strong> to the intake of micr<strong>on</strong>utrients. Ifcompositi<strong>on</strong>al parameters in nati<strong>on</strong>al regulati<strong>on</strong> do not accommodate the fortifiedimports (e.g. if the minimum ir<strong>on</strong> fortificati<strong>on</strong> level set by a newlyintroducedlaw was higher than the ir<strong>on</strong> c<strong>on</strong>tent of the imported product),an unintended diminuti<strong>on</strong> of micr<strong>on</strong>utrient supply may occur, unless thedomestic industry can quickly fill the gap.Regulators also need to be aware of the present level of community nutriti<strong>on</strong>alknowledge and any planned nutriti<strong>on</strong> educati<strong>on</strong> initiatives, so as to be ableto determine the appropriate balance between label informati<strong>on</strong> and educati<strong>on</strong>,and the type and amount of informati<strong>on</strong> required or permitted in labelling andadvertising. In this regard, and as previously indicated, regulators also need tobear in mind their obligati<strong>on</strong> to internati<strong>on</strong>al trade agreements and to internati<strong>on</strong>alstandards (see secti<strong>on</strong> 11.1).Finally, any amendment of a <strong>food</strong> law that requires industry to change its producti<strong>on</strong>practices and/or product labelling should incorporate a transiti<strong>on</strong>period. It inevitably takes some time before all domestic manufacturers andimporters become aware of new regulatory requirements and are able to modifytheir producti<strong>on</strong> and/or labelling operati<strong>on</strong>s accordingly. It may also be appropriatefor <strong>food</strong>s produced in accordance <strong>with</strong> a previous versi<strong>on</strong> of the law toc<strong>on</strong>tinue to be sold for a given period.11.3 Mandatory fortificati<strong>on</strong>If a <strong>food</strong> product is subject to mandatory fortificati<strong>on</strong>, then the manufacturer islegally obliged to add <strong>on</strong>e or more micr<strong>on</strong>utrients to that <strong>food</strong>. Mandatory fortificati<strong>on</strong>can reach the general populati<strong>on</strong> or an identified target group, depending<strong>on</strong> the c<strong>on</strong>sumpti<strong>on</strong> pattern of that <strong>food</strong>. For instance, fortificati<strong>on</strong> of a staple<strong>food</strong>, such as wheat flour, would increase the majority of the populati<strong>on</strong>’s intakeof a fortificant micr<strong>on</strong>utrient, whereas fortificati<strong>on</strong> of, say, formula infantmilk or complementary <strong>food</strong>s would increase the micr<strong>on</strong>utrient intake of a243
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSspecific populati<strong>on</strong> group <strong>on</strong>ly. The c<strong>on</strong>diti<strong>on</strong>s appropriate to the selecti<strong>on</strong>of mandatory fortificati<strong>on</strong> as either a populati<strong>on</strong> wide (mass) or specificpopulati<strong>on</strong> group (target) interventi<strong>on</strong> were discussed in Chapter 2 of these<str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>.Mandatory fortificati<strong>on</strong> is written into <strong>food</strong> law, usually in the form of regulati<strong>on</strong>which specifies a legal minimum, and where appropriate, a legal maximumlevel for each micr<strong>on</strong>utrient in the identified <strong>food</strong> or category of <strong>food</strong>s. Providingthere are no technological impediments, <strong>on</strong>e <strong>food</strong> or category of <strong>food</strong>s couldbe required to c<strong>on</strong>tain several added micr<strong>on</strong>utrients.This tends to apply to <strong>food</strong>stargeted at specific populati<strong>on</strong> groups having multiple nutriti<strong>on</strong>al needs andwhose <strong>food</strong> variety may be limited.11.3.1 Compositi<strong>on</strong>In its simplest form, a regulatory requirement governing the compositi<strong>on</strong> of afortified <strong>food</strong> might be written as follows:[Nominated <strong>food</strong>] must [c<strong>on</strong>tain]:(i) no less than [x] mg/kg of [micr<strong>on</strong>utrient name],and, where appropriate(ii) no more than [y] mg/kg of [micr<strong>on</strong>utrient name].Each of the key terms (i.e. those in italic typeface) are discussed in more detailbelow, <strong>with</strong> particular reference to the implicati<strong>on</strong>s for, and possible approachesto, mandatory regulati<strong>on</strong>.11.3.1.1 The nominated <strong>food</strong>The name of the <strong>food</strong> or <strong>food</strong> category selected for fortificati<strong>on</strong> must be generallyand unambiguously understood, or else explicitly defined or described inthe regulati<strong>on</strong>. The identity of the selected <strong>food</strong>(s) should corresp<strong>on</strong>d to the<strong>food</strong>(s) used to derive the level of fortificati<strong>on</strong> required to achieve pre-set programmenutriti<strong>on</strong>al goals (see secti<strong>on</strong> 7.3). Matching as closely as possible tothe identity of the <strong>food</strong>s used in the calculati<strong>on</strong>s enables more accurate predicti<strong>on</strong>sof programme impact <strong>on</strong> micr<strong>on</strong>utrient intake to be made.Potential areas of ambiguity or difficulty to be aware of include thefollowing:• The definiti<strong>on</strong> of a <strong>food</strong> or a <strong>food</strong> category may be as broad or as narrow asrequired. For example, the nominated <strong>food</strong> could simply be given as “flour”,which might mean all flours derived from all types of grain available in acountry. Alternatively, a much narrower descripti<strong>on</strong> could be employed, forinstance, “all flour from <strong>on</strong>e or more [specified] grains”, or “flour having [par-244
11. NATIONAL FOOD LAWticular compositi<strong>on</strong>al characteristics]” (which might be defined by extracti<strong>on</strong>rates); or “flour destined for [a particular use], such as bread making.• Where necessary, regulati<strong>on</strong>s should stipulate whether they apply to <strong>food</strong>ssold <strong>on</strong>ly at the retail level, or <strong>on</strong>ly at the wholesale level (for use as ingredientsin processed <strong>food</strong>s), or both. However, more precise descripti<strong>on</strong>s of<strong>food</strong>s or <strong>food</strong> categories, in the form of say, “<strong>food</strong> ingredient destined for [aparticular purpose]”, for example, bread-making flour or table salt, will automaticallydetermine the market level at which the bulk of the product is sold.• If necessary, the use of mandatorily fortified wholesale ingredients in certainprocessed <strong>food</strong>s could be more precisely c<strong>on</strong>trolled by stipulating that suchingredients either should always be or should never used be in particular<strong>food</strong>s, depending <strong>on</strong> the level of dietary intake of a given micr<strong>on</strong>utrient thatfortificati<strong>on</strong> is designed to achieve.11.3.1.2 “C<strong>on</strong>tain” or similar termThe term “c<strong>on</strong>tain”, or some such similar term, refers to the total amount ofmicr<strong>on</strong>utrient in the <strong>food</strong>. In other words, the legal minimum and maximumlevels apply to the amount of both naturally-occurring and fortificant micr<strong>on</strong>utrientpresent in a <strong>food</strong>, not just to the amount of fortificant that is added. Thisapproach suits those micr<strong>on</strong>utrients whose different chemical forms have similarbioavailabilities; more complex regulati<strong>on</strong> is needed in cases where there are significantdifferences in bioavailability between naturally-occurring and fortificantforms of the micr<strong>on</strong>utrient in questi<strong>on</strong>.Food manufacturers can adopt slightly different strategies for calculating theamount of micr<strong>on</strong>utrient that needs to be added in order to exceed the minimumrequirement depending <strong>on</strong> whether or not a maximum level is also establishedby regulati<strong>on</strong>. In cases where <strong>on</strong>ly a minimum requirement is set, and providingthat the cost of the fortificant is not prohibitive, manufacturers can ignore a<strong>food</strong>’s natural c<strong>on</strong>tent of a given micr<strong>on</strong>utrient, thus risking exceeding the legalminimum by at least the natural c<strong>on</strong>tent. However, if a total maximum level ofmicr<strong>on</strong>utrient is also prescribed, the <strong>food</strong>’s natural c<strong>on</strong>tent must be taken intoaccount to ensure the total does not exceed the maximum permissible limit. Incases where the natural c<strong>on</strong>tent is likely to be negligible, the legal minimum (x)and maximum levels (y) approximate to the range of permitted micr<strong>on</strong>utrientadditi<strong>on</strong>.11.3.1.3 Legal minimum and maximum levelsProcedures for determining the legal minimum (x) and maximum (y) totalmicr<strong>on</strong>utrient c<strong>on</strong>tent of a fortified <strong>food</strong> are set out in Chapter 7 of these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>.In c<strong>on</strong>ceptual terms, legal minimum levels are established <strong>on</strong> the basis of245
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSefficacy, whereas maximum levels are determined <strong>on</strong> the basis of safety or othermore c<strong>on</strong>servative criteria. Both the legal minimum and the maximum levelserve to protect human health, and thus could be used to justify any restricti<strong>on</strong>s<strong>on</strong> trade under the relevant internati<strong>on</strong>al trade agreements.Sometimes manufacturers need to add extra amounts of micr<strong>on</strong>utrient (anoverage) to account for any subsequent losses of fortificant during producti<strong>on</strong>,storage and distributi<strong>on</strong>, thereby ensuring that the <strong>food</strong> meets at least the legalminimum at the relevant distributi<strong>on</strong> point. When calculating overages, manufacturersshould bear in mind any maximum levels that may also apply to the<strong>food</strong> at that same distributi<strong>on</strong> point.The regulatory limits (i.e. the minimum and maximum levels) represent theextremes of the total permitted micr<strong>on</strong>utrient c<strong>on</strong>tent of the fortified <strong>food</strong> at thepoint(s) in the distributi<strong>on</strong> chain to which the regulati<strong>on</strong> applies. Generally thisis taken to be at the point of retail sale. Theoretically then, no individual <strong>food</strong>sample taken for testing from a retail outlet should have a micr<strong>on</strong>utrient c<strong>on</strong>tentoutside of these boundaries. However, as explained elsewhere in these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>,in some countries regulatory m<strong>on</strong>itoring or enforcement policies may allowa small defined deviati<strong>on</strong> or tolerance from the legal requirements as appropriateto the prevailing c<strong>on</strong>diti<strong>on</strong>s (see Chapter 8).11.3.1.4 Name of micr<strong>on</strong>utrientThe term used to identify the added micr<strong>on</strong>utrient can have significant ramificati<strong>on</strong>sfor both manufacturers and those involved in related m<strong>on</strong>itoring activities.Usually the generic name of the micr<strong>on</strong>utrient, for example, “iodine”, isused; this generally corresp<strong>on</strong>ds to that which is measured in laboratory analysisfor m<strong>on</strong>itoring purposes. However, most analytical methods employed in the<strong>food</strong> c<strong>on</strong>trol system do not discriminate between naturally-occurring and fortificantforms of a micr<strong>on</strong>utrient (a notable excepti<strong>on</strong> being folic acid).Many commercial micr<strong>on</strong>utrient fortificants c<strong>on</strong>tain other chemical entitiesthat c<strong>on</strong>tribute to the molecular weight (MW) of the compound. For example,iodine is commercially available as potassium iodate (KIO 3 , MW = 214), ofwhich about 60% is iodine, or as potassium iodide (KI, MW = 166), of whichabout 76% is iodine. A regulatory requirement expressed as “mg/kg of [micr<strong>on</strong>utrientname]”, refers to the amount of micr<strong>on</strong>utrient (i.e. iodine), not to theamount of the chemical compound (e.g. potassium iodate).This form of expressi<strong>on</strong>thus ensures that the same amount of the actual micr<strong>on</strong>utrient is added,irrespective of the chemical compositi<strong>on</strong> of the fortificant compound used. Forexample, salt fortificati<strong>on</strong> <strong>with</strong> iodine at a level of 20 mg iodine/kg of salt (assuminga negligible natural c<strong>on</strong>tent) requires the additi<strong>on</strong> of about 34 mg of potassiumiodate or about 26 mg of potassium iodide per kg of salt.246
11. NATIONAL FOOD LAWTABLE 11.1Relati<strong>on</strong>ship between legal minimum and maximum levels for ir<strong>on</strong> <strong>with</strong> regardto its relative bioavailability from selected fortificantsMineral compound Legal minimum level Maximum levelFerrous sulfate Natural ir<strong>on</strong> c<strong>on</strong>tent Natural ir<strong>on</strong> c<strong>on</strong>tent+ +Minimum amount ir<strong>on</strong> fromMaximum amount ir<strong>on</strong> fromferrous sulfateferrous sulfateElectrolytic ir<strong>on</strong> a Natural ir<strong>on</strong> c<strong>on</strong>tent Natural ir<strong>on</strong> c<strong>on</strong>tent+ +2 × Minimum amount ir<strong>on</strong> 2 × Maximum amount ir<strong>on</strong>specified for ferrous sulfate specified for ferrous sulfateaThe bioavailability of ir<strong>on</strong> from electrolytic ir<strong>on</strong> is approximately half that of ir<strong>on</strong> ferrous sulfate,so twice as much needs to added to deliver an equivalent amount of ir<strong>on</strong>.11.3.1.5 Permitted micr<strong>on</strong>utrient compoundsBecause commercially available fortificant compounds vary in their chemicalcompositi<strong>on</strong> and bioavailability, not all compounds are appropriate for use in all<strong>food</strong>s (see Part III). This gives rise to a number of opti<strong>on</strong>s for regulators: regulati<strong>on</strong>scan either include a list of all the permitted micr<strong>on</strong>utrient fortificantcompounds (leaving the <strong>food</strong> manufacturer free to chose which particular compoundto use), or it can permit the use of specific compounds in given categoriesof <strong>food</strong>s. Regulati<strong>on</strong>s can go further and stipulate the identity and purityrequirements of the permitted compounds, or make reference to pharmacopoeiasand other technical publicati<strong>on</strong>s that set out such requirements.For some micr<strong>on</strong>utrients, most notably ir<strong>on</strong>, significant differences in thebioavailability of the various ir<strong>on</strong>-c<strong>on</strong>taining chemical compounds can affect theefficacy of fortificati<strong>on</strong> and thus the amount of fortificant that needs to be added(see secti<strong>on</strong> 5.1). Table 11.1 shows how the legal minimum and maximum levelsof total ir<strong>on</strong> can be expressed in order to account for significant differences inthe relative bioavailability of ir<strong>on</strong> from the added fortificant compounds throughthe use of multiples of a reference amount. In this example, the minimum andmaximum amounts for ferrous sulfate are given by the sum of naturallyoccurringir<strong>on</strong> and ir<strong>on</strong> that is c<strong>on</strong>tributed by the added ferrous sulfate.Regulatory amounts applicable to the sec<strong>on</strong>d compound, electrolytic ir<strong>on</strong>, arecalculated assuming the same base amount of naturally-occurring ir<strong>on</strong> butdouble the amount of ir<strong>on</strong> from ferrous sulfate, ir<strong>on</strong> being the more bioavailablefrom the latter.11.3.2 Labelling and advertisingThe purpose of a <strong>food</strong> labelling is to identify the <strong>food</strong> inside the package andto provide the c<strong>on</strong>sumer <strong>with</strong> informati<strong>on</strong> about the <strong>food</strong> and its appropriate247
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTShandling and use. Basic informati<strong>on</strong> such as product name; “use by” or “bestbefore” date; storage instructi<strong>on</strong>s and directi<strong>on</strong>s for use; and ingredient list is asfor all <strong>food</strong>s and is not discussed further in these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>. In this c<strong>on</strong>textc<strong>on</strong>siderati<strong>on</strong> may be given to the Codex General Standard for the Labelling ofPre-packaged Foods (383).In the case of fortified <strong>food</strong>s, governments may establish regulati<strong>on</strong>s <strong>on</strong>labelling, claims and advertising requiring manufacturers to provide certainnutriti<strong>on</strong>al informati<strong>on</strong> to c<strong>on</strong>sumers. The usefulness and detail of such informati<strong>on</strong>will depend <strong>on</strong> the level of nutriti<strong>on</strong>al knowledge of target c<strong>on</strong>sumers,the assigned role of the label in fulfilling educati<strong>on</strong>al objectives of the fortificati<strong>on</strong>programme and the cost-effectiveness of this approach compared <strong>with</strong> alternativecommunicati<strong>on</strong> strategies.11.3.2.1 Micr<strong>on</strong>utrient declarati<strong>on</strong>How much qualitative or quantitative nutriti<strong>on</strong>al informati<strong>on</strong>, such as a standardizedlisting of the nutrient c<strong>on</strong>tent of a fortified <strong>food</strong>, to include <strong>on</strong> the label(apart from any reference to the micr<strong>on</strong>utrient in the name of the <strong>food</strong> such as“iodized” or “ir<strong>on</strong>-enriched/fortified” or its declarati<strong>on</strong> as a fortificant ingredient)is an important decisi<strong>on</strong> for regulators. Such decisi<strong>on</strong>s should be made inthe c<strong>on</strong>text of the target populati<strong>on</strong>’s nutriti<strong>on</strong>al knowledge and future nutriti<strong>on</strong>educati<strong>on</strong> initiatives. For instance, symbols or pictorial presentati<strong>on</strong>s, rather thanquantitative informati<strong>on</strong>, may be more efficacious am<strong>on</strong>g target populati<strong>on</strong>s <strong>with</strong>a high illiteracy rate and/or comparatively little knowledge of nutriti<strong>on</strong>. The costburden of providing nutriti<strong>on</strong>al informati<strong>on</strong>, initially borne by the manufacturerbut subsequently passed to the c<strong>on</strong>sumer, is another factor to c<strong>on</strong>sider. SeveralCodex texts provide general guidance regarding labelling and claims and maybe helpful to regulators; these are the Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong> Labelling(342) and the Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong> Claims (343) (see alsoAnnex F).Quantitative micr<strong>on</strong>utrient declarati<strong>on</strong> requirements can pose a particularchallenge to manufacturers and regulators because of the labile nature of somemicr<strong>on</strong>utrients <strong>with</strong> time. In many regulatory systems, the veracity of label informati<strong>on</strong>applies to the product at the point of sale; external m<strong>on</strong>itoring for compliancealso tends to occur at this stage. Specific menti<strong>on</strong> of such matters ismade in secti<strong>on</strong> 3.5 of the Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong> Labelling (342). Regulatorsmay also wish to c<strong>on</strong>sider the need for “best before” dates <strong>on</strong> l<strong>on</strong>g shelflifefortified <strong>food</strong>s, especially if the n<strong>on</strong>-fortified versi<strong>on</strong>s are exempt from datemarking (e.g. solid sugars or <strong>food</strong> grade salt). Stipulating a best-before date providesa means of linking the nutrient declarati<strong>on</strong> to the shelf-life period.248
11. NATIONAL FOOD LAW11.3.2.2 Nutriti<strong>on</strong> and health-related claimsClaims are statements that manufacturers voluntarily make to inform c<strong>on</strong>sumersabout their products. Nutriti<strong>on</strong> and health-related claims focus <strong>on</strong> the nutriti<strong>on</strong>alproperties of the <strong>food</strong>, or its nutriti<strong>on</strong>al and, where permitted, health benefitsfor c<strong>on</strong>sumers. Nutriti<strong>on</strong> and health claims are especially relevant to voluntarilyfortified <strong>food</strong>s, and are discussed in more detail in the secti<strong>on</strong> <strong>on</strong> voluntaryfortificati<strong>on</strong> (see secti<strong>on</strong> 11.4.2). Two issues are, however, specific to mandatoryfortificati<strong>on</strong>. Although there is little incentive for manufacturers to voluntarilymake nutriti<strong>on</strong> and health-related claims about their products when all the <strong>food</strong>sin <strong>on</strong>e category are fortified, if the mandatorily fortified <strong>food</strong> c<strong>on</strong>stitutes <strong>on</strong>ly aporti<strong>on</strong> of the entire <strong>food</strong> category (e.g. table salt vis a vis all salt), then manufacturersmay choose to make lawful claims about the nutriti<strong>on</strong>al properties andpotential benefits of c<strong>on</strong>sumpti<strong>on</strong> of their fortified products. Under these circumstances,the issues for regulators are the same as for voluntary fortificati<strong>on</strong>(see secti<strong>on</strong> 11.4.2).Sec<strong>on</strong>dly, some mandatorily fortified raw ingredients are used in the manufactureof highly-processed energy-rich <strong>food</strong>s. The processed <strong>food</strong>s themselvesthus become fortified, albeit indirectly and to a lesser extent. Regulators mightwish to c<strong>on</strong>sider whether any restricti<strong>on</strong>s should be placed <strong>on</strong> the ability of indirectlyfortified processed <strong>food</strong>s to bear nutriti<strong>on</strong> and health-related claims thatrefer to, or are based <strong>on</strong>, the fortified nature of the product.11.3.3 Trade c<strong>on</strong>siderati<strong>on</strong>sPrescribing mandatory fortificati<strong>on</strong> requirements in regulati<strong>on</strong> may imposetrade restricti<strong>on</strong>s <strong>on</strong> imported products, either because they are unfortified orthey have been fortified differently. These trade restricti<strong>on</strong>s may cause difficultiesfor a country’s trading partners. Nevertheless, it is clear from WTO jurisprudencethat not <strong>on</strong>ly do countries have the right to determine the level of healthprotecti<strong>on</strong> they deem to be appropriate – providing such measures do not unnecessarilyrestrict trade – but also the protecti<strong>on</strong> of human health is <strong>on</strong>e of severallegitimate objectives that countries can cite in justificati<strong>on</strong> of a trade restricti<strong>on</strong>(see secti<strong>on</strong> 11.1) (384).Such c<strong>on</strong>siderati<strong>on</strong>s aside, different fortificati<strong>on</strong> requirements betweennati<strong>on</strong>s may well create some practical difficulties for intercountry trade. Nati<strong>on</strong>sin the same regi<strong>on</strong>, <strong>with</strong> similar public health nutriti<strong>on</strong> problems and <strong>food</strong> cultures,may benefit from finding a comm<strong>on</strong> positi<strong>on</strong> <strong>on</strong> fortificati<strong>on</strong> policy andregulati<strong>on</strong> that could be uniformally adopted. This would not <strong>on</strong>ly provide forintraregi<strong>on</strong>al trade and potential ec<strong>on</strong>omies of scale, but also increase the leverageof the regi<strong>on</strong>, where necessary, to source an imported fortified productaccording to the regi<strong>on</strong>’s particular specificati<strong>on</strong>s. Although, mandatorily fortified<strong>food</strong> moving in internati<strong>on</strong>al trade can be imported not <strong>on</strong>ly by countries249
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS<strong>with</strong> compatible mandatory fortificati<strong>on</strong> regulati<strong>on</strong>s but also by those countrieswhose voluntary fortificati<strong>on</strong> regulati<strong>on</strong>s accommodate the compositi<strong>on</strong> of theimported <strong>food</strong>, the product labelling may need to be modified so that it is nati<strong>on</strong>allycompliant. The need for labelling modificati<strong>on</strong> will depend <strong>on</strong> the flexibilityof the labelling requirements of the importing country.11.4 Voluntary fortificati<strong>on</strong>Voluntary fortificati<strong>on</strong> occurs when a manufacturer freely chooses to fortify<strong>food</strong>s. It is practised widely in most industrialized countries and increasingly indeveloping countries. The extent to which a manufacturer’s decisi<strong>on</strong> to fortifya <strong>food</strong> is voluntary and independent does, however, vary depending <strong>on</strong> themicr<strong>on</strong>utrient and the prevailing sociopolitical and legal envir<strong>on</strong>ment. In somecases, the impetus for voluntary fortificati<strong>on</strong> flows from government – in theform of incentives, collaborative arrangements or an expectati<strong>on</strong> of cooperati<strong>on</strong><strong>with</strong> specific voluntary fortificati<strong>on</strong> permissi<strong>on</strong>s – often as a milderalternative to mandatory fortificati<strong>on</strong>. In several industrialized countries, theregulati<strong>on</strong>s governing the fortificati<strong>on</strong> of some basic commodities, such assalt and margarine, represent examples of this particular brand of voluntaryfortificati<strong>on</strong>.More comm<strong>on</strong>ly, voluntary fortificati<strong>on</strong> is driven by a desire <strong>on</strong> the part ofindustry and the c<strong>on</strong>sumer to increase micr<strong>on</strong>utrient intake as a means ofobtaining possible health benefits. Perhaps not surprisingly, commercial c<strong>on</strong>siderati<strong>on</strong>sare frequently decisive factors in the development of voluntarily fortified<strong>food</strong> products. Such products are promoted, through labelling andadvertising, <strong>on</strong> the basis of their health and nutriti<strong>on</strong>al features.The proliferati<strong>on</strong> of fortified products that has occurred in recent years couldhave important implicati<strong>on</strong>s for future micr<strong>on</strong>utrient intakes and dietary habits.Most significantly, increased c<strong>on</strong>sumpti<strong>on</strong> of fortified products may result inintakes of certain micr<strong>on</strong>utrients that pose potential risks to public health.Therefore,governments are advised to exercise an appropriate degree of c<strong>on</strong>trol overvoluntary fortificati<strong>on</strong>, either in the form of <strong>food</strong> regulati<strong>on</strong> or through cooperativearrangements (e.g. a code of practice). The regulati<strong>on</strong> of voluntary fortificati<strong>on</strong>should not <strong>on</strong>ly be c<strong>on</strong>sistent <strong>with</strong> overall regulatory objectives, butshould also take account of the Codex General Principles for the Additi<strong>on</strong> ofEssential Nutrients to Foods (385) (see Annex F).As in the case of mandatory fortificati<strong>on</strong>, there are several key issues that needaddressing when developing regulati<strong>on</strong>s for voluntary fortificati<strong>on</strong>, in particular,issues which relate to the compositi<strong>on</strong>, labelling and advertising, and thetrade of fortified products. These are discussed in greater detail below, but inessence are as follows:250
11. NATIONAL FOOD LAW• the range of <strong>food</strong>s suitable for fortificati<strong>on</strong>;• the range and c<strong>on</strong>centrati<strong>on</strong>s of micr<strong>on</strong>utrients appropriate for different categoriesof <strong>food</strong>s;• the mode of regulatory expressi<strong>on</strong> (i.e. whether there is a need for absolutelimits or whether more flexible mechanisms for establishing compositi<strong>on</strong>alparameters would be more workable);• the identity of, and purity specificati<strong>on</strong>s for, the listed fortificant compounds;• c<strong>on</strong>trols <strong>on</strong> nutriti<strong>on</strong>al and health claims as well as advertising, and the appropriatelevel of detail of nutriti<strong>on</strong> label informati<strong>on</strong>.11.4.1 Compositi<strong>on</strong>11.4.1.1 Range of <strong>food</strong>sThere is c<strong>on</strong>siderable debate and certainly no internati<strong>on</strong>al c<strong>on</strong>sensus regardingthe extent to which regulators should seek to minimize public health risksdue to MNM, particularly in relati<strong>on</strong> to the range of <strong>food</strong>s that are eligible forvoluntary fortificati<strong>on</strong>. To date, the debate has centred <strong>on</strong> whether the choice of<strong>food</strong>s or <strong>food</strong> categories for voluntary fortificati<strong>on</strong> should be decided by governments,or left entirely to manufacturers, in which case the prevailing technologicaland/or commercial c<strong>on</strong>straints – such as whether the fortificantadversely affects product characteristics or the cost is dissuasive or prohibitive– will largely determine which products are fortified and which are not.One view is that, <strong>with</strong>out some regulatory c<strong>on</strong>straint, the proliferati<strong>on</strong> andpromoti<strong>on</strong> of fortified <strong>food</strong>s has the potential to modify <strong>food</strong> choices and dietarybehaviour in ways that are not commensurate <strong>with</strong> the maintenance of healthand well-being.These c<strong>on</strong>cerns anticipate that the commercial promoti<strong>on</strong> of voluntarilyfortified <strong>food</strong>s would enhance their appeal to c<strong>on</strong>sumers who wouldexpect to gain a health benefit from the c<strong>on</strong>sumpti<strong>on</strong> of such <strong>food</strong>s. Furthermore,if c<strong>on</strong>sumers resp<strong>on</strong>ded regularly to such promoti<strong>on</strong>al activities, this couldlead to dietary distorti<strong>on</strong>s in which fortified <strong>food</strong>s are favoured over naturallynutritious <strong>food</strong>s. It might also c<strong>on</strong>found c<strong>on</strong>sumers’ percepti<strong>on</strong> and understandingof the nutriti<strong>on</strong>al c<strong>on</strong>tributi<strong>on</strong> of various <strong>food</strong>s to a healthful diet, andthus undermine efforts to educate them about the nutriti<strong>on</strong>al value of different<strong>food</strong>s and the importance of a varied diet for ensuring adequate intakes of essentialnutrients. Collectively, these influences may have a detrimental effect <strong>on</strong> thequantity, quality and ratio of intakes of certain macr<strong>on</strong>utrients, and thus c<strong>on</strong>stitutea l<strong>on</strong>g-term health risk for the populati<strong>on</strong>.Of greater c<strong>on</strong>cern is the possibility that some promoted fortified <strong>food</strong>s willc<strong>on</strong>tain relatively high quantities of nutrients that are associated <strong>with</strong> negativehealth effects, in particular, total fat, saturated and trans-fatty acids, sodium or251
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSsalt, sugar(s) and alcohol. The <strong>food</strong>s most incriminated are those that nutriti<strong>on</strong>policies often advise against regular c<strong>on</strong>sumpti<strong>on</strong>, such as c<strong>on</strong>fecti<strong>on</strong>ery, carb<strong>on</strong>atedsoft drinks, sugar-based beverages and desserts, high-salt and high-fatsnacks and alcoholic beverages.At present, the c<strong>on</strong>cern about the proliferati<strong>on</strong> of fortified <strong>food</strong>s is largelybased <strong>on</strong> predicti<strong>on</strong>s about future market evoluti<strong>on</strong>, which are supported by theobservati<strong>on</strong> that manufacturers often use the fact that a <strong>food</strong> is fortified as apromoti<strong>on</strong>al tool. Those who support a liberal approach to the regulati<strong>on</strong> of voluntaryfortificati<strong>on</strong> cite the lack of evidence from any industrialized country thathas a well-developed nutriti<strong>on</strong> educati<strong>on</strong> system for such an evoluti<strong>on</strong> and pastexperience <strong>with</strong> a liberal approach to the additi<strong>on</strong> of micr<strong>on</strong>utrients. Accordingto manufacturers’ data, voluntarily fortified <strong>food</strong>s currently represent 1–6% ofthe total <strong>food</strong> supply in such countries, a percentage that has remained stablein recent years. There is also little c<strong>on</strong>crete evidence of any negative effect offortified <strong>food</strong>s <strong>on</strong> the overall balance of populati<strong>on</strong> micr<strong>on</strong>utrient intake. Suchfindings suggest that the key factors to c<strong>on</strong>sider when deciding the extent of permissi<strong>on</strong>sfor voluntary fortificati<strong>on</strong> are the strength and sustainability of nutriti<strong>on</strong>educati<strong>on</strong> programmes, the level of c<strong>on</strong>sumers’ nutriti<strong>on</strong>al knowledge andthe potential for c<strong>on</strong>sumer c<strong>on</strong>fusi<strong>on</strong>.The nutriti<strong>on</strong>al profile of candidate <strong>food</strong>s, in particular their c<strong>on</strong>tent of totalfat, saturated and trans-fatty acids, sugar(s), sodium or salt, is clearly <strong>on</strong>e criteriathat could be used to select appropriate <strong>food</strong>s for voluntary fortificati<strong>on</strong>.However, a flexible approach that also c<strong>on</strong>siders the nutriti<strong>on</strong>al merits of a candidate<strong>food</strong> will avoid the inadvertent exclusi<strong>on</strong> of nutriti<strong>on</strong>ally valuable <strong>food</strong>sfrom potential fortificati<strong>on</strong>.When reviewing candidate <strong>food</strong>s <strong>with</strong> respect to theirnutriti<strong>on</strong>al value, reference should be made to the recently published report ofthe Joint FAO/WHO Expert C<strong>on</strong>sultati<strong>on</strong> <strong>on</strong> Diet, Nutriti<strong>on</strong> and the Preventi<strong>on</strong>of Chr<strong>on</strong>ic Diseases (386). However, it is recognized that final decisi<strong>on</strong>sabout the suitability of <strong>food</strong>s for fortificati<strong>on</strong> will very much depend <strong>on</strong> thedietary profile and nutriti<strong>on</strong>al status of the populati<strong>on</strong> and so will vary fromcountry to country. In c<strong>on</strong>trast to the requirements for mandatory fortificati<strong>on</strong>(see secti<strong>on</strong> 11.3.1.1), the range of <strong>food</strong>s eligible for voluntary fortificati<strong>on</strong> caneither be c<strong>on</strong>sidered to be prohibited unless permitted (i.e. a positive list), orpermitted unless prohibited (i.e. a negative list). If the risks to health from unsafefortificati<strong>on</strong> are c<strong>on</strong>siderable, it is probably preferable to establish a positiverather than a negative list of <strong>food</strong>s.11.4.1.2 The range of micr<strong>on</strong>utrients and their specific chemical formsA review of the balance between the public health significance and public healthrisk of individual micr<strong>on</strong>utrients is generally c<strong>on</strong>sidered to be a suitable basisfor drawing up a list of micr<strong>on</strong>utrients that would be appropriate to add to <strong>food</strong>s252
11. NATIONAL FOOD LAWthrough voluntary fortificati<strong>on</strong>. Globally, the micr<strong>on</strong>utrients of greatest publichealth significance are ir<strong>on</strong>, vitamin A and iodine. A number of other micr<strong>on</strong>utrientsalso offer broad public health benefits or potential benefits to smallerpopulati<strong>on</strong> groups (see Chapter 4). There may be some micr<strong>on</strong>utrients,however, whose additi<strong>on</strong> to <strong>food</strong>s may not necessarily c<strong>on</strong>fer any further publichealth benefit because of the surfeit of that micr<strong>on</strong>utrient in the existing <strong>food</strong>supply, in which case, fortificati<strong>on</strong> serves <strong>on</strong>ly to promote the “image” of theproduct. Some would argue that <strong>on</strong>e micr<strong>on</strong>utrient more or less would not haveany significant impact <strong>on</strong> c<strong>on</strong>sumer percepti<strong>on</strong> of the product, and that thereforethese micr<strong>on</strong>utrients should be approved, providing there were no safetyc<strong>on</strong>cerns.Ideally, the public health risks of individual micr<strong>on</strong>utrients should be assessedin terms of the magnitude of the difference between some measure of nutrientadequacy and an upper safe intake limit. In recent years, a number of scientificbodies have proposed various risk classificati<strong>on</strong> systems for micr<strong>on</strong>utrients,many of which have much in comm<strong>on</strong> (93). For example, thiamine is comm<strong>on</strong>lyrated as low risk whereas selenium is rated as high risk. The classificati<strong>on</strong> ofmicr<strong>on</strong>utrients as moderate to high risk does not preclude their regulatoryapproval, particularly as these same micr<strong>on</strong>utrients may provide significant benefits;however, it does signal the need for their additi<strong>on</strong> to <strong>food</strong>s to be carefullyregulated. It may also be necessary to make provisi<strong>on</strong> for a small number of c<strong>on</strong>stituents,such as vanadium, whose status as essential nutrients is uncertain atthe present time. In this regard, it should be noted that the definiti<strong>on</strong> of fortificati<strong>on</strong>given in the Codex General Principles for the Additi<strong>on</strong> of Essential Nutrientsto Foods (385) refers specifically to the additi<strong>on</strong> of essential nutrients. Theoverriding c<strong>on</strong>siderati<strong>on</strong> must, however, be to ensure the adequate nutriti<strong>on</strong>albalance of the diet.Having decided <strong>on</strong> the range of approved micr<strong>on</strong>utrients, regulators areadvised to include these in regulati<strong>on</strong> in the form of a restrictive list. Furtherrefinements of the permissi<strong>on</strong>s or prohibiti<strong>on</strong>s <strong>on</strong> particular <strong>food</strong>-micr<strong>on</strong>utrientcombinati<strong>on</strong>s can then be developed from this primary list. A related restrictivelist of particular micr<strong>on</strong>utrient fortificant compounds (i.e. the vitamin preparati<strong>on</strong>sand mineral salts that are used as sources of vitamins and minerals), wouldalso be required. Regulators should bear in mind that the potential range of <strong>food</strong>sthat can be voluntarily fortified is large, and so too is the range of <strong>food</strong> producti<strong>on</strong>methods. Therefore, the list of approved chemical compounds will needto be as large as the basic criteria for selecti<strong>on</strong> (i.e. bioavailability, safety) willallow. Purity criteria for these compounds will also need to be stipulated. Thesecan be developed at the nati<strong>on</strong>al level but the task is arduous and resource intensive.Purity criteria have been set for most substances at the internati<strong>on</strong>al leveland so reference to texts such as the Food Chemicals Codex (387) and the Britishpharmacopoeia (388) could be made instead.253
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS11.4.1.3 Legal minimum and maximum levelsThere are two issues to be c<strong>on</strong>sidered here: firstly, the setting of minimum andmaximum levels and, sec<strong>on</strong>dly, the amount of <strong>food</strong> that will be used as a referencefor these levels (i.e. mg per kg or per serving).Minimum micr<strong>on</strong>utrient levels should set such that fortificati<strong>on</strong> results inproducts that c<strong>on</strong>tain a meaningful amount of the micr<strong>on</strong>utrient, that is to say,amounts that would be expected to c<strong>on</strong>tribute a benefit when that product wasc<strong>on</strong>sumed in quantities that would normally be expected as part of an overalladequate and varied diet. An alternative approach, which provides greater flexibilityfor manufacturers (and also importers), is to establish minimum claim criteria).When deciding which is the more suitable approach, regulators shouldtake into account the health benefits that are likely to be gained from voluntaryfortificati<strong>on</strong>.The setting of maximum levels is a more complex matter because of thenecessity to simultaneously eliminate potential risks to public health from excessintakes of certain nutrients and to preserve the balance of the nutriti<strong>on</strong>al compositi<strong>on</strong>of the diet. Decisi<strong>on</strong>s about the appropriate maximum limits formicr<strong>on</strong>utrients in <strong>food</strong>s eligible for voluntary fortificati<strong>on</strong> should be based <strong>on</strong>dietary intake assessments that take account of intakes from all dietary sourcesof the micr<strong>on</strong>utrient under c<strong>on</strong>siderati<strong>on</strong>, including that from unfortified <strong>food</strong>sand dietary supplements. However, this does not necessarily mean thatmaximum amounts need to be established for all micr<strong>on</strong>utrients according totheir risk profile: not <strong>on</strong>ly would this be difficult to do for the full range ofmicr<strong>on</strong>utrients, but the risk of excess intake varies <strong>with</strong> the micr<strong>on</strong>utrient and<strong>with</strong> the level of deficiency (and so will be different for different populati<strong>on</strong>s).Neither does it mean that the maximum amounts need to be set at the estimatedhighest safe level in each fortified <strong>food</strong> category. Allowance would need to bemade for the applicability of the upper limits (particularly for the at-risk group),the assumpti<strong>on</strong>s made in the dietary assessment (e.g. that supplement use wouldnot become more prevalent), and the magnitude of future intakes of micr<strong>on</strong>utrientsfrom fortified <strong>food</strong>.A risk-based approach for setting maximum fortificati<strong>on</strong> limits is becomingmore comm<strong>on</strong>ly accepted, particularly <strong>with</strong> the development of reference valuesfor upper safe intakes, the approach followed by others is that officially recommendednutrient intakes, i.e. a populati<strong>on</strong> measure of dietary adequacy, variouslyabbreviated in different countries as the DRI, the RDI, the RNI or theDRV, are better guiding criteria. The reas<strong>on</strong>ing behind the latter suggesti<strong>on</strong>stems from acknowledgement of the absence of need for higher intakes andgreater compatibility <strong>with</strong> the amounts of micr<strong>on</strong>utrients found naturally in<strong>food</strong>s.254
11. NATIONAL FOOD LAWIt is apparent from the preceding discussi<strong>on</strong> that it would be unwise to allowthe additi<strong>on</strong> of those micr<strong>on</strong>utrients that have a narrow margin of safety in significantquantities to all or even a wide range of <strong>food</strong>s. Therefore, the range of<strong>food</strong>s to which they may be added should be prioritized or restricted in someway; this can be d<strong>on</strong>e <strong>on</strong> the basis of their nature and importance in the diet ofthe general populati<strong>on</strong> or of certain populati<strong>on</strong> groups. Regulators who administersystems in which the <strong>food</strong>s are restrictively listed and may be incrementallyapproved through petiti<strong>on</strong> should give c<strong>on</strong>siderati<strong>on</strong> ahead of time, if possible,to the most appropriate <strong>food</strong> sources of these micr<strong>on</strong>utrients.Maximum levels can be established in regulati<strong>on</strong> either for all added micr<strong>on</strong>utrients,or just for those micr<strong>on</strong>utrients that are associated <strong>with</strong> a known risk,according to the level of risk. As in the case of minimum levels, the c<strong>on</strong>cept ofmaximum claim levels may be advantageous. The rati<strong>on</strong>ale behind the use ofmaximum claims is that they allow regulators to set restricti<strong>on</strong>s <strong>on</strong> the maximummicr<strong>on</strong>utrient levels that are proporti<strong>on</strong>ate to the level of risk and, in the absenceof a tolerance system, they allow manufacturers (and particularly importers)more latitude in deciding the micr<strong>on</strong>utrient compositi<strong>on</strong> of <strong>food</strong>s lawfullyoffered for sale. However, for domestic manufacturers, commercial reality alsoimposes its own c<strong>on</strong>straints in that the manufacturer gains no market advantageby adding c<strong>on</strong>siderably more fortificant than the amount that can be claimed.As indicated above, the quantitative basis for setting minimum and maximummicr<strong>on</strong>utrient levels is a very important c<strong>on</strong>siderati<strong>on</strong>. There are three possibilitiesthat would uniformly apply to all eligible <strong>food</strong>s. These are:— maximum c<strong>on</strong>centrati<strong>on</strong> per unit weight or volume (e.g. per 100 g or ml);— maximum micr<strong>on</strong>utrient density per unit energy (i.e. per 100 kcal or kJ);— maximum quantity per nominated serving or reference quantity (e.g. g orml per serving).The use of weight- or energy-based criteria requires making assumpti<strong>on</strong>s aboutrespective amounts of solids and liquids, or energy ingested by an average c<strong>on</strong>sumerin <strong>on</strong>e day. Since these are likely to be broadly similar across nati<strong>on</strong>alpopulati<strong>on</strong>s, the potential exists for agreement at the regi<strong>on</strong>al or internati<strong>on</strong>allevel, providing the basic approach is acceptable. On the down side, both theweight- and energy-based criteria would cause some products to be undulyfavoured or penalized (e.g. energy-rich or low-energy <strong>food</strong>s, <strong>food</strong>s used in smallquantities) so that excepti<strong>on</strong>s would need to be made accordingly. The perserving basis has the attracti<strong>on</strong> of being more relevant for c<strong>on</strong>sumers, especiallyif the label nutrient declarati<strong>on</strong> is made <strong>on</strong> the same basis. However, it necessitatesagreement <strong>on</strong> the serving size, which is more likely to vary am<strong>on</strong>g countriesaccording to cultural <strong>food</strong> patterns. Agreement <strong>on</strong> serving size would thus255
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSbe more difficult to reach at an internati<strong>on</strong>al level, and therefore setting levels<strong>on</strong> this basis would be more likely to create problems for internati<strong>on</strong>al trade.11.4.2 Labelling and advertisingAs previously menti<strong>on</strong>ed, claims regarding the nutriti<strong>on</strong>al properties of fortified<strong>food</strong>, or its nutriti<strong>on</strong>al and, where permitted, health benefits for c<strong>on</strong>sumers arefrequently made by manufacturers as a means of promoting their products; thisis particularly true of voluntarily fortified <strong>food</strong>s. Examples of nutriti<strong>on</strong>al propertyclaims are those which refer to a <strong>food</strong> “c<strong>on</strong>taining” or being a “source” or“high source” of a particular nutrient and those which compare the nutrientc<strong>on</strong>tent of a <strong>food</strong> <strong>with</strong> <strong>on</strong>e or more <strong>food</strong>s. Health-related claims include nutrientfuncti<strong>on</strong> and reducti<strong>on</strong> of disease risk claims, i.e. they refer to the relati<strong>on</strong>shipbetween a nutrient (or a special ingredient c<strong>on</strong>tained in the <strong>food</strong>) andnormal physiological functi<strong>on</strong>s of the body or to the reducti<strong>on</strong> in risk of adisease, including nutrient deficiency diseases.11.4.2.1 Nutriti<strong>on</strong> and health-related claimsAppropriate regulati<strong>on</strong> of claims ensures that the informati<strong>on</strong> manufacturersc<strong>on</strong>vey to c<strong>on</strong>sumers about their products is truthful and not misleading.The Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong> Claims (343) provides guidanceto governments <strong>on</strong> the c<strong>on</strong>diti<strong>on</strong>s for nutriti<strong>on</strong> and health-related claims andestablishes the general principle that these claims should be c<strong>on</strong>sistent <strong>with</strong> andsupport nati<strong>on</strong>al nutriti<strong>on</strong> policy. At the time of writing, c<strong>on</strong>diti<strong>on</strong>s for the useof health claims are under discussi<strong>on</strong>. Regulating claims about the reducti<strong>on</strong> ofrisk of disease is an especially challenging task and should be tackled <strong>with</strong>extreme cauti<strong>on</strong>. Regulators should bear in mind that anything less than a caseby-caseassessment and detailed evaluati<strong>on</strong> of adequately substantiated requestsfrom manufacturers to use disease reducti<strong>on</strong> claims would need to be carefullyc<strong>on</strong>sidered.The Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong> Claims (343) recommend thatclaims should be substantiated by generally acceptable scientific data, althoughthe meaning of “generally acceptable scientific data” can give rise to differentinterpretati<strong>on</strong>s. A list of health claims that are c<strong>on</strong>sidered to be well establishedand generally acceptable would be a useful tool both for the resp<strong>on</strong>sible manufacturerand for <strong>food</strong> c<strong>on</strong>trolling authorities. Ideally, a procedure that allowsupdates to be made <strong>with</strong>in an agreed time frame should be integral to sucha list.As an alternative to a list of approved health claims, nutriti<strong>on</strong> and healthrelatedclaims may be c<strong>on</strong>trolled by setting qualifying and disqualifying criteriathat are based <strong>on</strong> other aspects of the <strong>food</strong>. Currently-held views <strong>on</strong> this topichave much in comm<strong>on</strong> <strong>with</strong> those previously described in relati<strong>on</strong> to the range256
11. NATIONAL FOOD LAWof candidate <strong>food</strong>s for voluntary fortificati<strong>on</strong>. Although it is reas<strong>on</strong>able to expectthat all eligible voluntarily fortified <strong>food</strong>s should also be eligible to carry nutriti<strong>on</strong>and health-related claims, this approach may introduce discrepanciesbetween the criteria that apply to fortified <strong>food</strong>s and those that apply to unfortified<strong>food</strong>s, particularly if <strong>food</strong>s not permitted direct fortificati<strong>on</strong> are made fromfortified ingredients. It is therefore useful to c<strong>on</strong>sider whether the qualifying criteriafor claims for fortified <strong>food</strong>s should differ from those that would apply tounfortified <strong>food</strong>s (whose claims are based <strong>on</strong> a natural micr<strong>on</strong>utrient c<strong>on</strong>tent),and if so, <strong>on</strong> what basis.11.4.2.2 Micr<strong>on</strong>utrient declarati<strong>on</strong>Because of the positive percepti<strong>on</strong> of fortificati<strong>on</strong> by the c<strong>on</strong>sumer, manufacturersusually wish to promote this aspect of their products by making nutrientc<strong>on</strong>tent and/or other related claims about their product. This generally triggersnutriti<strong>on</strong> labelling of the <strong>food</strong>. Even in the absence of a claim, manufacturersmay choose to declare micr<strong>on</strong>utrient c<strong>on</strong>tents in nutriti<strong>on</strong> labelling.Provisi<strong>on</strong> of (usually quantitative) informati<strong>on</strong> about nutrient c<strong>on</strong>tents is normallyrequired under the rules <strong>on</strong> nutriti<strong>on</strong> labelling; this coupled <strong>with</strong> informati<strong>on</strong>about the micr<strong>on</strong>utrient(s) <strong>with</strong> which the product is fortified, would bea minimum requirement. For those c<strong>on</strong>sumers who read and understand nutriti<strong>on</strong>labelling, the declarati<strong>on</strong> of the fortificant micr<strong>on</strong>utrient c<strong>on</strong>tent in thenutriti<strong>on</strong> informati<strong>on</strong> panel could potentially enhance the “image” of the <strong>food</strong>.It should therefore be c<strong>on</strong>sidered whether more comprehensive nutriti<strong>on</strong> informati<strong>on</strong>should be given for fortified <strong>food</strong>s in order to provide more balancedinformati<strong>on</strong> about the product.The Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong> Labelling (342) provides guidance togovernments <strong>on</strong> nutriti<strong>on</strong> labelling.11.4.2.3 Other relevant c<strong>on</strong>siderati<strong>on</strong>sThe labelling and advertising of fortified products should not attribute to themundue nutriti<strong>on</strong>al merits. It should also avoid c<strong>on</strong>veying the impressi<strong>on</strong> that anormal balanced and varied diet would not provide adequate quantities of nutrients,although regulati<strong>on</strong>s should allow for the possibility of scientifically substantiatedexcepti<strong>on</strong>s to this. Allowing additi<strong>on</strong>al advice <strong>on</strong> the need for abalanced diet is an another opti<strong>on</strong> to c<strong>on</strong>sider.11.4.3 Trade c<strong>on</strong>siderati<strong>on</strong>sVoluntary fortificati<strong>on</strong> regulati<strong>on</strong>, despite being less restrictive than that governingmandatory fortificati<strong>on</strong>, may nevertheless limit trade in fortified <strong>food</strong>sbetween countries, particularly in cases where the micr<strong>on</strong>utrient c<strong>on</strong>centrati<strong>on</strong>s257
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSof fortified <strong>food</strong>s do not c<strong>on</strong>form to the regulatory provisi<strong>on</strong>s of the importingcountry, or where fortificati<strong>on</strong> of a <strong>food</strong> category is not permitted or is prohibitedin the importing country. Different labelling regulati<strong>on</strong>s, including thosegoverning nutriti<strong>on</strong> labelling and claims, may mean that product labels wouldhave to be adapted to local requirements. If a comm<strong>on</strong> language exists, forreas<strong>on</strong>s of cost and efficiency, it would be preferable to harm<strong>on</strong>ize regi<strong>on</strong>al regulati<strong>on</strong>s.This would bring the added b<strong>on</strong>us of minimizing such impediments totrade.258
References1. Ir<strong>on</strong> deficiency anaemia: assessment, preventi<strong>on</strong>, and c<strong>on</strong>trol. A guide for programmemanagers. Geneva, World Health Organizati<strong>on</strong>, 2001 (WHO/NHD/01.3).2. de Benoist B et al., eds. Iodine status worldwide.WHO Global Database <strong>on</strong> IodineDeficiency. Geneva, World Health Organizati<strong>on</strong>, 2004.3. Global Prevalence of Vitamin A Deficiency. Micr<strong>on</strong>utrient Deficiency Informati<strong>on</strong>System working paper No. 2. Geneva, World Health Organizati<strong>on</strong>, 1995 (WHO/NUT/95.3.).4. The World Health Report 2002: reducing risks, promoting healthy life: overview. Geneva,World Health Organizati<strong>on</strong>, 2002 (WHO/WHR/02.1).5. Indicators for assessing vitamin A deficiency and their applicati<strong>on</strong> in m<strong>on</strong>itoring andevaluating interventi<strong>on</strong> programmes. Geneva, World Health Organizati<strong>on</strong>, 1996(WHO/NUT/96.10).6. Assessment of iodine deficiency disorders and m<strong>on</strong>itoring their eliminati<strong>on</strong>. A guide forprogramme managers. 2nd ed. Geneva, World Health Organizati<strong>on</strong>, 2001.7. Allen LH. Ending hidden hunger: the history of micr<strong>on</strong>utrient deficiency c<strong>on</strong>trol.Washingt<strong>on</strong>, DC, The World Bank, 2002 (Background Paper for the WorldBank/UNICEF Nutriti<strong>on</strong> Assessment).8. Hetzel BS, Pandav CS. S.O.S. for a Billi<strong>on</strong>. The C<strong>on</strong>quest of Iodine Deficiency Disorders.Oxford, Oxford University Press, 1994.9. Hetzel BS. Iodine deficiency disorders and their eradiacti<strong>on</strong>. Lancet, 1983,2:1126–1129.10. Cobra C et al. Infant survival is improved by oral iodine supplementati<strong>on</strong>. Journalof Nutriti<strong>on</strong>, 1997, 127:574–578.11. Thilly CH et al. Impaired fetal and postnatal development and high perinatal deathratein a severe iodine deficient area. In: Stockigt JR et al., eds. Thyroid ResearchVIII. Canberra, Australian Academy of Science, 1980: 20–23.12. Beat<strong>on</strong> GH et al. Effectiveness of vitamin A supplementati<strong>on</strong> in the c<strong>on</strong>trol of youngchild morbidity and mortality in developing countries. Geneva, Administrative Committee<strong>on</strong> Coordinati<strong>on</strong> – Sub-Committee <strong>on</strong> Nutriti<strong>on</strong>, 1992 (ACC/SCN Nutriti<strong>on</strong>policy paper No. 13).13. Sommer A et al. Impact of vitamin A supplementati<strong>on</strong> <strong>on</strong> childhood mortality. Arandomized c<strong>on</strong>trolled community trial. Lancet, 1986, 1:1169–1173.14. Haas JD, Brownlie T. Ir<strong>on</strong> deficiency and reduced work capacity: a critical reviewof the research to determine a causal relati<strong>on</strong>ship. Journal of Nutriti<strong>on</strong>, 2001, 131(2S-2):676S–688S.15. Pollitt E. The developmental and probabilistic nature of the functi<strong>on</strong>al c<strong>on</strong>sequencesof ir<strong>on</strong>-deficiency anemia in children. Journal of Nutriti<strong>on</strong>, 2001,131:669S–675S.259
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS16. Stoltzfus RJ. Ir<strong>on</strong>-deficiency anemia: reexamining the nature and magnitude of thepublic health problem. Summary: implicati<strong>on</strong>s for research and programs. Journalof Nutriti<strong>on</strong>, 2001, 131:697S–701S.17. Brown KH et al. Effect of supplemental zinc <strong>on</strong> the growth and serum zinc c<strong>on</strong>centrati<strong>on</strong>sof prepubertal children: a meta-analysis of randomized c<strong>on</strong>trolled trials.American Journal of Clinical Nutriti<strong>on</strong>, 2002, 75:1062–1071.18. Bhutta ZA et al. Preventi<strong>on</strong> of diarrhea and pneum<strong>on</strong>ia by zinc supplementati<strong>on</strong>in children in developing countries: pooled analysis of randomized c<strong>on</strong>trolled trials.Zinc Investigators’ Collaborative Group. Journal of Pediatrics, 1999, 135:689–697.19. Black RE. Therapeutic and preventive effects of zinc <strong>on</strong> serious childhood infectiousdiseases in developing countries. American Journal of Clinical Nutriti<strong>on</strong>, 1998,68 (2 Suppl):476S–479S.20. Resoluti<strong>on</strong> WHA 43.2. Preventi<strong>on</strong> and c<strong>on</strong>trol of iodine deficiency disorders. In:Forty-third World Health Assembly, Geneva 14 May 1990. Geneva, World HealthOrganizati<strong>on</strong>, 1990.21. Demment MW, Allen LH, eds. Animal Source Foods to Improve Micr<strong>on</strong>utrientNutriti<strong>on</strong> and Human Functi<strong>on</strong> in Developing Countries. Proceedings of the c<strong>on</strong>ferenceheld in Washingt<strong>on</strong>, DC, 2002 June 24–26. Journal of Nutriti<strong>on</strong>, 2003, 133(11 Suppl 2):3875S–4061S.22. de Pee S, Bloem MW, Kiess L. Evaluating <strong>food</strong>-based programmes for their reducti<strong>on</strong>of vitamin A deficiency and its c<strong>on</strong>sequences. Food and Nutriti<strong>on</strong> Bulletin, 2000,21:232–238.23. Gibs<strong>on</strong> RS et al. Dietary strategies to combat micr<strong>on</strong>utrient deficiencies of ir<strong>on</strong>,zinc, and vitamin A in developing countries: Development, implementati<strong>on</strong>, m<strong>on</strong>itoring,and evaluati<strong>on</strong>. Food and Nutriti<strong>on</strong> Bulletin, 2000, 21:219–231.24. Ruel MT. Can <strong>food</strong>-based strategies help reduce vitamin A and ir<strong>on</strong> deficiencies? Areview of recent evidence. Washingt<strong>on</strong>, DC, Internati<strong>on</strong>al Food Policy Research Institute,2001.25. Burgi H, Supersaxo Z, Selz B. Iodine deficiency diseases in Switzerland <strong>on</strong>ehundred years after Theodor Kocher’s survey: a historical review <strong>with</strong> some newgoitre prevalence data. Acta Endocrinologica, 1990, 123:577–590.26. Marine D, Kimball OP. Preventi<strong>on</strong> of simple goiter in man. Archives of Internal Medicine,1920, 25:661–672.27. Darnt<strong>on</strong>-Hill I, Nalubola R. Fortificati<strong>on</strong> strategies to meet micornutrient needs:successes and failures. Proceedings of the Nutriti<strong>on</strong> Society, 2002, 61:231–241.28. Thuy PV et al. Regular c<strong>on</strong>sumpti<strong>on</strong> of NaFeEDTA-fortified fish sauce improvesir<strong>on</strong> status and reduces the prevalence of anemia in anemic Vietnamese women.American Journal of Clinical Nutriti<strong>on</strong>, 2003, 78:284–290.29. Mannar V, Boy Gallego E. Ir<strong>on</strong> fortificati<strong>on</strong>: country level experiences and less<strong>on</strong>slearned. Journal of Nutriti<strong>on</strong>, 2002, 132 (4 Suppl):856S–858S.30. Ballot DE et al. Fortificati<strong>on</strong> of curry powder <strong>with</strong> NaFe(111)EDTA in an ir<strong>on</strong>deficientpopulati<strong>on</strong>: report of a c<strong>on</strong>trolled ir<strong>on</strong>-fortificati<strong>on</strong> trial. American Journalof Clinical Nutriti<strong>on</strong>, 1989, 49:162–169.31. Muhilal et al. Vitamin A-fortified m<strong>on</strong>osodium glutamate and health, growth, andsurvival of children: a c<strong>on</strong>trolled field trial. American Journal of Clinical Nutriti<strong>on</strong>,1988, 48:1271–1276.32. Sol<strong>on</strong> FS et al. Evaluati<strong>on</strong> of the effect of vitamin A-fortified margarine <strong>on</strong> thevitamin A status of preschool Filipino children. European Journal of Clinical Nutriti<strong>on</strong>,1996, 50:720–723.260
REFERENCES33. Sol<strong>on</strong> FS et al. Efficacy of a vitamin A-fortified wheat-flour bun <strong>on</strong> the vitamin Astatus of Filipino schoolchildren. American Journal of Clinical Nutriti<strong>on</strong>, 2000,72:738–744.34. van Stuijvenberg ME et al. L<strong>on</strong>g-term evaluati<strong>on</strong> of a micr<strong>on</strong>utrient-fortified biscuitused for addressing micr<strong>on</strong>utrient deficiencies in primary school children. PublicHealth Nutriti<strong>on</strong>, 2001, 4:1201–1209.35. Latham MC et al. Micr<strong>on</strong>utrient dietary supplements – a new fourth approach.Archivos Latinoamericanos de Nutrici<strong>on</strong>, 2001, 51 (1 Suppl 1):37–41.36. Abrams SA et al. A micr<strong>on</strong>utrient-fortified beverage enhances the nutriti<strong>on</strong>al statusof children in Botswana. Journal of Nutriti<strong>on</strong>, 2003, 133:1834–1840.37. Yip R et al. Declining prevalence of anemia in childhood in a middle-class setting:a pediatric success story? Pediatrics, 1987, 80:330–334.38. Fom<strong>on</strong> S. Infant feeding in the 20th century: formula and beikost. Journal of Nutriti<strong>on</strong>,2001, 131:409S–420S.39. Layrisse M et al. Early resp<strong>on</strong>se to the effect of ir<strong>on</strong> fortificati<strong>on</strong> in the Venezuelanpopulati<strong>on</strong>. American Journal of Clinical Nutriti<strong>on</strong>, 1996, 64:903–907.40. Stekel A et al. Preventi<strong>on</strong> of ir<strong>on</strong> deficiency by milk fortificati<strong>on</strong>. II. A field trial <strong>with</strong>a full-fat acidified milk. American Journal of Clinical Nutriti<strong>on</strong>, 1988, 47:265–269.41. Hertrampf E. Ir<strong>on</strong> fortificati<strong>on</strong> in the Americas. Nutriti<strong>on</strong> Reviews, 2002,60:S22–S25.42. <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for ir<strong>on</strong> fortificati<strong>on</strong> of cereal <strong>food</strong> staples.Washingt<strong>on</strong>, DC, Sharing UnitedStates Technology to Aid in the Improvement of Nutriti<strong>on</strong>, 2001.43. Zimmermann MB et al. Additi<strong>on</strong> of microencapsulated ir<strong>on</strong> to iodized salt improvesthe efficacy of iodine in goitrous, ir<strong>on</strong>-deficient children: a randomized, doubleblind,c<strong>on</strong>trolled trial. European Journal of Endocrinology, 2002, 147:747–753.44. Zimmermann MB et al. Dual fortificati<strong>on</strong> of salt <strong>with</strong> iodine and microencapsulatedir<strong>on</strong>: a randomized, double-blind, c<strong>on</strong>trolled trial in Moroccan schoolchildren.American Journal of Clinical Nutriti<strong>on</strong>, 2003, 77:425–432.45. Arroyave G et al. Evaluati<strong>on</strong> of sugar fortificati<strong>on</strong> <strong>with</strong> vitamin A at the nati<strong>on</strong>al level.Washingt<strong>on</strong>, DC, Pan American Health Organizati<strong>on</strong>, 1979 (Scientific publicati<strong>on</strong>No. 384).46. Arroyave G, Mejia LA, Aguilar JR. The effect of vitamin A fortificati<strong>on</strong> of sugar <strong>on</strong>the serum vitamin A levels of preschool Guatemalan children: a l<strong>on</strong>gitudinal evaluati<strong>on</strong>.American Journal of Clinical Nutriti<strong>on</strong>, 1981, 34:41–49.47. Arroyave G et al. Effectos del c<strong>on</strong>sumo de azucar fortifacada c<strong>on</strong> retinol,por la madre embarazada y lactante cuya dieta habitual es baja en vitamin A.Estudio de la madre y del nino. [Effects of the intake of sugar fortified <strong>with</strong> retinol,by the pregnant women and infant whose diet is usually low in vitamin A.Study of the mother and child]. Archivos Latinoamericanos de Nutrici<strong>on</strong>, 1974,24:485–512.48. H<strong>on</strong>ein MA et al. Impact of folic acid fortificati<strong>on</strong> of the US <strong>food</strong> supply <strong>on</strong> theoccurrence of neural tube defects. Journal of the American Medical Associati<strong>on</strong>, 2001,285:2981–2986.49. Jacques PF et al. The effect of folic acid fortificati<strong>on</strong> <strong>on</strong> plasma folate and totalhomocysteine c<strong>on</strong>centrati<strong>on</strong>s. New England Journal of Medicine, 1999, 340:1449–1454.50. Lewis CJ et al. Estimated folate intakes: data updated to reflect <strong>food</strong> fortificati<strong>on</strong>,increased bioavailability, and dietary supplement use. American Journal of ClinicalNutriti<strong>on</strong>, 1999, 70:198–207.261
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS51. Ray JG et al. Associati<strong>on</strong> of neural tube defects and folic acid <strong>food</strong> fortificati<strong>on</strong> inCanada. Lancet, 2002, 360:2047–2048.52. Hirsch S et al. The Chilean flour folic acid fortificati<strong>on</strong> program reduces serumhomocysteine levels and masks vitamin B-12 deficiency in elderly people. Journalof Nutriti<strong>on</strong>, 2002, 132:289–291.53. Ray JG et al. Persistence of vitamin B12 insufficiency am<strong>on</strong>g elderly women afterfolic acid <strong>food</strong> fortificati<strong>on</strong>. Clinical Biochemistry, 2003, 36:387–391.54. Park YK et al. Effectiveness of <strong>food</strong> fortificati<strong>on</strong> in the United States: the case ofpellagra. American Journal of Public Health, 2000, 90:727–738.55. Welch TR, Bergstrom WH,Tsang RC.Vitamin D-deficient rickets: the reemergenceof a <strong>on</strong>ce-c<strong>on</strong>quered disease. Journal of Pediatrics, 2000, 137:143–145.56. Nesby-O’Dell S et al. Hypovitaminosis D prevalence and determinants am<strong>on</strong>gAfrican American and white women of reproductive age: third Nati<strong>on</strong>al Health andNutriti<strong>on</strong> Examinati<strong>on</strong> Survey, 1988–1994. American Journal of Clinical Nutriti<strong>on</strong>,2002, 76:187–192.57. Keane EM et al. Vitamin D-fortified liquid milk: benefits for the elderly community-basedpopulati<strong>on</strong>. Calcified Tissue Internati<strong>on</strong>al, 1998, 62:300–302.58. Kinyamu HK et al. Dietary calcium and vitamin D intake in elderly women: effect<strong>on</strong> serum parathyroid horm<strong>on</strong>e and vitamin D metabolites. American Journal ofClinical Nutriti<strong>on</strong>, 1998, 67:342–348.59. Enriching lives: overcoming vitamin and mineral malnutriti<strong>on</strong> in developing countries.Washingt<strong>on</strong>, DC, World Bank, 1994.60. Hort<strong>on</strong> S. Opportunities for investment in nutriti<strong>on</strong> in low-income Asia. AsianDevelopment Review, 1999, 17:246–273.61. Codex Alimentarius Commissi<strong>on</strong>. General Principles for the Additi<strong>on</strong> of EssentialNutrients to Foods CAC/GL 09-1987 (amended 1989, 1991). Rome, Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commisi<strong>on</strong>, 1987(http://www.codexalimentarius.net/download/standards/299/CXG_009e.pdf,accessed 7 October 2005).62. Beat<strong>on</strong> GH. Fortificati<strong>on</strong> of <strong>food</strong>s for refugee feeding. Final report to the Canadian Internati<strong>on</strong>alDevelopment Agency. Ontario, GHB C<strong>on</strong>sulting, 1995.63. Department of Health. Nutriti<strong>on</strong> and b<strong>on</strong>e health. Report of the subgroup <strong>on</strong> b<strong>on</strong>ehealth, working group <strong>on</strong> the nutriti<strong>on</strong>al status of the populati<strong>on</strong> of the Committee <strong>on</strong>Medical Aspects of Food and Nutriti<strong>on</strong> Policy. L<strong>on</strong>d<strong>on</strong>, The Stati<strong>on</strong>ery Office, 1998.64. Gibs<strong>on</strong> SA. Ir<strong>on</strong> intake and ir<strong>on</strong> status of preschool children: associati<strong>on</strong>s<strong>with</strong> breakfast cereals, vitamin C and meat. Public Health Nutriti<strong>on</strong>, 1999, 2:521–528.65. Nestel P et al. Complementary <strong>food</strong> supplements to achieve micr<strong>on</strong>utrient adequacyfor infants and young children. Journal of Pediatric Gastroenterology andNutriti<strong>on</strong>, 2003, 36:316–328.66. Zlotkin S et al. Treatment of anemia <strong>with</strong> microencapsulated ferrous fumarate plusascorbic acid supplied as sprinkles to complementary (weaning) <strong>food</strong>s. AmericanJournal of Clinical Nutriti<strong>on</strong>, 2001, 74:791–795.67. Briend A. Highly nutrient-dense spreads: a new approach to delivering multiplemicr<strong>on</strong>utrients to high-risk groups. British Journal of Nutriti<strong>on</strong>, 2001, 85 (Suppl2):175–179.68. Ministry of Health and Child Welfare and CARE Internati<strong>on</strong>al. Report of Sub-Regi<strong>on</strong>al Workshop <strong>on</strong> Fortificati<strong>on</strong> at Hammermill Level; 2000 Nov 13–16; Harare,Zimbabwe. Harare, CARE Internati<strong>on</strong>al Zimbabwe, 2000.262
REFERENCES69. Beyer P et al. Golden Rice: introducing the beta-carotene biosynthesis pathway intorice endosperm by genetic engineering to defeat vitamin A deficiency. Journal ofNutriti<strong>on</strong>, 2002, 132:506S–510S.70. Ye X et al. Engineering the provitamin A (beta-carotene) biosynthetic pathway into(carotenoid-free) rice endosperm. Science, 2000, 287:303–305.71. Lucca P, Hurrell R, Potrykus I. Fighting ir<strong>on</strong> deficiency anemia <strong>with</strong> ir<strong>on</strong>-rich rice.Journal of the American College of Nutriti<strong>on</strong>, 2002, 21 (3 Suppl):184S–190S.72. Safety aspects of genetically modified <strong>food</strong>s of plant origin. Report of a Joint FAO/WHOExpert C<strong>on</strong>sultati<strong>on</strong> <strong>on</strong> Foods Derived from Biotechnology,WHO Headquaters, Geneva,Switzerland, 29 May to 2 June 2000. Geneva, World Health Organizati<strong>on</strong>, 2000(WHO/SDE/PHE/FOS/00.6).73. Allen LH, Gillespie SR. What works? A review of the efficacy and effectiveness of nutriti<strong>on</strong>interventi<strong>on</strong>s. Geneva, Administrative Committee <strong>on</strong> Coordinati<strong>on</strong> – Sub-Committee <strong>on</strong> Nutriti<strong>on</strong>, 2001 (ACC/SCN State-of-the-Art Series, Nutriti<strong>on</strong>Policy Discussi<strong>on</strong> Paper No. 19).74. Assessing the ir<strong>on</strong> status of populati<strong>on</strong>s: report of a Joint World Health Organizati<strong>on</strong>/Centers for Disease C<strong>on</strong>trol and Preventi<strong>on</strong> Technical C<strong>on</strong>sultati<strong>on</strong> <strong>on</strong> the Assessment ofIr<strong>on</strong> Status at the Populati<strong>on</strong> Level, Geneva, Switzerland, 6–8 April 2004. Geneva,World Health Organizati<strong>on</strong>, 2005.75. Staubli Asobayire F et al. Prevalence of ir<strong>on</strong> deficiency <strong>with</strong> and <strong>with</strong>out c<strong>on</strong>currentanemia in populati<strong>on</strong> groups <strong>with</strong> high prevalences of malaria and otherinfecti<strong>on</strong>s: a study in Cote d’Ivoire. American Journal of Clinical Nutriti<strong>on</strong>, 2001,74:776–782.76. Menendez C, Fleming AF, Al<strong>on</strong>so PL. Malaria-related anaemia. Parasitology Today,2000, 16:469–476.77. Allen LH, Casterline-Sabel JE. Prevalence and causes of nutriti<strong>on</strong>al anemias. In:Ramakrishnan U, ed. Nutriti<strong>on</strong>al Anemias. Boca Rat<strong>on</strong>, FL, CRC Press, 2000: 17–21.78. Requirements of vitamin A, ir<strong>on</strong>, folate and vitamin B12. Report of a Joint FAO/WHOExpert C<strong>on</strong>sultati<strong>on</strong>. Rome, Food and Agriculture Organizati<strong>on</strong> of the UnitedNati<strong>on</strong>s, 1988 (FAO Food and Nutriti<strong>on</strong> Series, No. 23).79. De Maeyer EM et al. Preventing and c<strong>on</strong>trolling ir<strong>on</strong> deficiency anaemia throughprimary health care. A guide for health administrators and programme managers.Geneva, World Health Organizati<strong>on</strong>, 1989.80. Brownlie T et al. Marginal ir<strong>on</strong> deficiency <strong>with</strong>out anemia impairs aerobic adaptati<strong>on</strong>am<strong>on</strong>g previously untrained women. American Journal of Clinical Nutriti<strong>on</strong>,2002, 75:734–742.81. Brabin BJ, Hakimi M, Pelletier D. An analysis of anemia and pregnancy-relatedmaternal mortality. Journal of Nutriti<strong>on</strong>, 2001, 131 (2S-2):604S–614S.82. Brabin BJ, Premji Z, Verhoeff F. An analysis of anemia and child mortality. Journalof Nutriti<strong>on</strong>, 2001, 131 (2S-2):636S–645S.83. Cogswell ME et al. Ir<strong>on</strong> supplementati<strong>on</strong> during pregnancy, anemia, and birthweight: a randomized c<strong>on</strong>trolled trial. American Journal of Clinical Nutriti<strong>on</strong>, 2003,78:773–781.84. Rosales FJ et al. Ir<strong>on</strong> deficiency in young rats alters the distributi<strong>on</strong> of vitamin Abetween plasma and liver and between hepatic retinol and retinyl esters. Journal ofNutriti<strong>on</strong>, 1999, 129:1223–1228.85. Munoz EC et al. Ir<strong>on</strong> and zinc supplementati<strong>on</strong> improves indicators of vitamin Astatus of Mexican preschoolers. American Journal of Clinical Nutriti<strong>on</strong>, 2000,71:789–794.263
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS86. Zimmermann MB et al. Persistence of goiter despite oral iodine supplementati<strong>on</strong>in goitrous children <strong>with</strong> ir<strong>on</strong> deficiency anemia in Cote d’Ivoire. American Journalof Clinical Nutriti<strong>on</strong>, 2000, 71:88–93.87. Zimmermann MB. Ir<strong>on</strong> status influences the efficacy of iodine prophylaxis ingoitrous children in Cote d’Ivoire. Internati<strong>on</strong>al Journal of Vitamin and Nutriti<strong>on</strong>Research, 2002, 72:19–25.88. Sommer A, Davids<strong>on</strong> FR. Assessment and c<strong>on</strong>trol of vitamin A deficiency: theAnnecy Accords. Journal of Nutriti<strong>on</strong>, 2002, 132 (9 Suppl):2845S–2850S.89. West KP Jr. Extent of vitamin A deficiency am<strong>on</strong>g preschool children and womenof reproductive age. Journal of Nutriti<strong>on</strong>, 2002, 132 (9 Suppl):2857S–2866S.90. Allen LH, Haskell M. Vitamin A requirements of infants under six m<strong>on</strong>ths of age.Food and Nutriti<strong>on</strong> Bulletin, 2001, 22:214–234.91. Food and Nutriti<strong>on</strong> Board, Institute of Medicine. Dietary reference intakes for vitaminA, vitamin K, arsenic, bor<strong>on</strong>, chromium, copper, iodine, ir<strong>on</strong>, manganese, molybdenum,nickel, silic<strong>on</strong>, vanadium, and zinc.Washingt<strong>on</strong>, DC, Nati<strong>on</strong>al Academy Press, 2001.92. Miller M et al. Why do children become vitamin A deficient? Journal of Nutriti<strong>on</strong>,2002, 132 (9 Suppl):2867S–2880S.93. Vitamin and mineral requirements in human nutriti<strong>on</strong>. Report of a Joint FAO/WHOExpert C<strong>on</strong>sultati<strong>on</strong> <strong>on</strong> Human Vitamin and Mineral Requirements, Bangkok,Thailand,21–30 September 1998. 2nd ed. Geneva, World Health Organizati<strong>on</strong>, 2004.94. de Pee S,West CE. Dietary carotenoids and their role in combating vitamin A deficiency:a review of the literature. European Journal of Clinical Nutriti<strong>on</strong>, 1996, 50(Suppl 3):S38–S53.95. Rodriguez MS, Irwin MI. A c<strong>on</strong>spectus of research <strong>on</strong> vitamin A requirements ofman. Journal of Nutriti<strong>on</strong>, 1972, 102:909–968.96. Castenmiller JJ, West CE. Bioavailability and bioc<strong>on</strong>versi<strong>on</strong> of carotenoids. AnnualReview of Nutriti<strong>on</strong>, 1998, 18:19–38.97. West KP Jr. et al. Double blind, cluster randomised trial of low dose supplementati<strong>on</strong><strong>with</strong> vitamin A or beta carotene <strong>on</strong> mortality related to pregnancy inNepal. The NNIPS-2 Study Group. British Medical Journal, 1999, 318:570–575.98. Christian P et al. Night blindness during pregnancy and subsequent mortalityam<strong>on</strong>g women in Nepal: effects of vitamin A and beta-carotene supplementati<strong>on</strong>.American Journal of Epidemiology, 2000, 152:542–547.99. Suharno D et al. Supplementati<strong>on</strong> <strong>with</strong> vitamin A and ir<strong>on</strong> for nutriti<strong>on</strong>al anaemiain pregnant women in West Java, Ind<strong>on</strong>esia. Lancet, 1993, 342:1325–1328.100. Delange F. The disorders induced by iodine deficiency. Thyroid, 1994, 4:107–128.101. Delange F. Cassava and the thyroid. In: Gaitan E, ed. Envir<strong>on</strong>mental goitrogenesis.Boca Rat<strong>on</strong>, FL, CRC Press, 1989: 173–194.102. Delange F. Endemic cretinism. In: Braverman LE, Utiger RD, eds. The thyroid. Afundamental and clinical text. Philadelphia, Lippincott, 2000: 743–754.103. Stanbury JB, ed. The damaged brain of iodine deficiency: cognitive, behavioral,neuromotor, educative aspects. New York, Cognizant Communicati<strong>on</strong> Corporati<strong>on</strong>,1994.104. Bleichrodt N, Born MA. A meta-analysis of research <strong>on</strong> iodine and its relati<strong>on</strong>shipto cognitive development. In: Stanbury J, ed. The damaged brain of iodine deficiency:cognitive, behavioral, neuromotor, and educative aspects. New York, Cognizant Communicati<strong>on</strong>Corporati<strong>on</strong>, 1994: 195–200.105. Boyages SC. Clinical review 49: Iodine deficiency disorders. Journal of ClinicalEndocrinology and Metabolism, 1993, 77:587–591.264
REFERENCES106. Delange F et al. Iodine deficiency in the world: where do we stand at the turn ofthe century? Thyroid, 2001, 11:437–447.107. Osendarp SJ, West CE, Black RE. The need for maternal zinc supplementati<strong>on</strong> indeveloping countries: an unresolved issue. Journal of Nutriti<strong>on</strong>, 2003, 133:817S–827S.108. Sian L et al. Zinc homeostasis during lactati<strong>on</strong> in a populati<strong>on</strong> <strong>with</strong> a low zincintake. American Journal of Clinical Nutriti<strong>on</strong>, 2002, 75:99–103.109. Holt C, Brown KH, eds. Internati<strong>on</strong>al Zinc Nutriti<strong>on</strong> C<strong>on</strong>sultative Group(IZiNCG) Technical Document #1. Assessment of the risk of zinc deficiency inpopulati<strong>on</strong>s and opti<strong>on</strong>s for its c<strong>on</strong>trol. Food and Nutriti<strong>on</strong> Bulletin, 2004, 25 (Suppl2):S94–S203.110. Sandström B. Dietary pattern and zinc supply. In: Mills CF, ed. Zinc in humanbiology. New York, Springer-Verlag, 1989: 350–365.111. Sandström B, L<strong>on</strong>nerdal B. Promoters and antag<strong>on</strong>ists of zinc absorpti<strong>on</strong>. In: MillsCF, ed. Zinc in human biology. New York, Springer-Verlag, 1989: 57–78.112. Sandström B et al. Effect of protein level and protein source <strong>on</strong> zinc absorpti<strong>on</strong> inhumans. Journal of Nutriti<strong>on</strong>, 1989, 119:48–53.113. Sian L et al. Zinc absorpti<strong>on</strong> and intestinal losses of endogenous zinc in youngChinese women <strong>with</strong> marginal zinc intakes. American Journal of Clinical Nutriti<strong>on</strong>,1996, 63:348–353.114. Petters<strong>on</strong> DS, Sandström B, Cederblad Å. Absorpti<strong>on</strong> of zinc from lupin (Lupinusangustifolius)-based <strong>food</strong>s. British Journal of Nutriti<strong>on</strong>, 1994, 72:865–871.115. Davidss<strong>on</strong> L et al. Dietary fiber in weaning cereals: a study of the effect <strong>on</strong> stoolcharacteristics and absorpti<strong>on</strong> of energy, nitrogen, and minerals in healthy infants.Journal of Pediatric Gastroenterology and Nutriti<strong>on</strong>, 1996, 22:167–179.116. Manary MJ et al. Zinc homeostasis in Malawian children c<strong>on</strong>suming a highphytate,maize-based diet. American Journal of Clinical Nutriti<strong>on</strong>, 2002, 75:1057–1061.117. Hambidge M. Human zinc deficiency. Journal of Nutriti<strong>on</strong>, 2000, 130 (5S Suppl):1344S–1349S.118. Shankar AH et al. The influence of zinc supplementati<strong>on</strong> <strong>on</strong> morbidity due toPlasmodium falciparum: a randomized trial in preschool children in PapuaNew Guinea. American Journal of Tropical Medicine and Hygiene, 2000, 62:663–669.119. Muller O et al. Effect of zinc supplementati<strong>on</strong> <strong>on</strong> malaria and other causes of morbidityin west African children: randomised double blind placebo c<strong>on</strong>trolled trial.British Medical Journal, 2001, 322:1567.120. Caulfield LE et al. Potential c<strong>on</strong>tributi<strong>on</strong> of maternal zinc supplementati<strong>on</strong> duringpregnancy to maternal and child survival. American Journal of Clinical Nutriti<strong>on</strong>,1998, 68 (2 Suppl):499S–508S.121. Brent<strong>on</strong> DP, Jacks<strong>on</strong> MJ,Young A. Two pregnancies in a patient <strong>with</strong> acrodermatitisenteropathica treated <strong>with</strong> zinc sulphate. Lancet, 1981, 2:500–502.122. King JC. Determinants of maternal zinc status during pregnancy. American Journalof Clinical Nutriti<strong>on</strong>, 2000, 71 (5 Suppl):1334S–1343S.123. Merialdi M et al. Adding zinc to prenatal ir<strong>on</strong> and folate tablets improves fetalneurobehavioral development. American Journal of Obstetrics and Gynecology, 1999,180:483–490.124. Caulfield LE et al. Maternal zinc supplementati<strong>on</strong> does not affect size at birth orpregnancy durati<strong>on</strong> in Peru. Journal of Nutriti<strong>on</strong>, 1999, 129:1563–1568.265
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS125. Sazawal S et al. Zinc supplementati<strong>on</strong> in infants born small for gestati<strong>on</strong>al agereduces mortality: a prospective, randomized, c<strong>on</strong>trolled trial. Pediatrics, 2001,108:1280–1286.126. Domellof M et al. Ir<strong>on</strong>, zinc, and copper c<strong>on</strong>centrati<strong>on</strong>s in breast milk are independentof maternal mineral status. American Journal of Clinical Nutriti<strong>on</strong>, 2004,79:111–115.127. Krebs NF et al. Zinc supplementati<strong>on</strong> during lactati<strong>on</strong>: effects <strong>on</strong> maternal status andmilk zinc c<strong>on</strong>centrati<strong>on</strong>s. American Journal of Clinical Nutriti<strong>on</strong>, 1995, 61:1030–1036.128. Food and Nutriti<strong>on</strong> Board, Institute of Medicine. Dietary reference intakes forthiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin,and choline. Washingt<strong>on</strong>, DC, Nati<strong>on</strong>al Academy Press, 2000.129. Rucker RB et al. Handbook of vitamins. 3rd ed. New York, Marcel Dekker, 2001.130. Review of the magnitude of Folate and Vitamin B12 deficiencies worldwide. McLean E,de Benoist B, Allen LH, 2005.131. Krishnaswamy K, Madhavan Nair K. Importance of folate in human nutriti<strong>on</strong>.British Journal of Nutriti<strong>on</strong>, 2001, 85 (Suppl 2):115–124.132. Hertrampf E et al. C<strong>on</strong>sumpti<strong>on</strong> of folic acid-fortified bread improves folate statusin women of reproductive age in Chile. Journal of Nutriti<strong>on</strong>, 2003, 133:3166–3169.133. Villapando S et al. Vitamins A and C and folate status in Mexican children under12 years and women 12–49 years: A probabilistic nati<strong>on</strong>al survey. Salud Publica deMexico, 2003, 45 (Suppl 4):S508–S519.134. Koebnick C et al. Folate status during pregnancy in women is improved by l<strong>on</strong>gtermhigh vegetable intake compared <strong>with</strong> the average western diet. Journal of Nutriti<strong>on</strong>,2001, 131:733–739.135. Charoenlarp P et al. A WHO collaborative study <strong>on</strong> ir<strong>on</strong> supplementati<strong>on</strong> in Burmaand in Thailand. American Journal of Clinical Nutriti<strong>on</strong>, 1988, 47:280–297.136. Berry RJ et al. Preventi<strong>on</strong> of neural-tube defects <strong>with</strong> folic acid in China. NewEngland Journal of Medicine, 1999, 341:1485–1490.137. Werler MM, Shapiro S, Mitchell AA. Peric<strong>on</strong>cepti<strong>on</strong>al folic acid exposure and riskof occurrent neural tube defects. Journal of the American Medical Associati<strong>on</strong>, 1993,269:1257–1261.138. Botto LD et al. Neural-tube defects. New England Journal of Medicine, 1999,341:1509–1519.139. Shibuya K, Murray CJL. C<strong>on</strong>genital anomalies. In: Murray CJL, Lopez AD, eds.Health dimensi<strong>on</strong>s of sex and reproducti<strong>on</strong>. Bost<strong>on</strong>, Harvard University Press, 1998:455–512.140. Moyers S, Bailey LB. Fetal malformati<strong>on</strong>s and folate metabolism: review of recentevidence. Nutriti<strong>on</strong> Reviews, 2001, 59:215–224.141. de Onis M, Villar J, Gulmezoglu M. Nutriti<strong>on</strong>al interventi<strong>on</strong>s to prevent intrauterinegrowth retardati<strong>on</strong>: evidence from randomized c<strong>on</strong>trolled trials. EuropeanJournal of Clinical Nutriti<strong>on</strong>, 1998, 52 (Suppl 1):S83–S93.142. Wald NJ et al. Homocysteine and ischemic heart disease: results of a prospectivestudy <strong>with</strong> implicati<strong>on</strong>s regarding preventi<strong>on</strong>. Archives of Internal Medicine, 1998,158:862–867.143. Perry IJ et al. Prospective study of serum total homocysteine c<strong>on</strong>centrati<strong>on</strong> and riskof stroke in middle-aged British men. Lancet, 1995, 346:1395–1398.144. De Bree A et al. Homocysteine determinants and the evidence to what extent homocysteinedetermines the risk of cor<strong>on</strong>ary heart disease. Pharmacological Reviews,2002, 54:599–618.266
REFERENCES145. Wald DS, Law M, Morris JK. Homocysteine and cardiovascular disease: evidence<strong>on</strong> causality from a meta-analysis. British Medical Journal, 2002, 325:1202–1206.146. Malouf M, Grimley EJ, Areosa SA. Folic acid <strong>with</strong> or <strong>with</strong>out vitamin B12 for cogniti<strong>on</strong>and dementia. The Cochrane Database of Systematic Reviews, 2003, Issue 4.Art. No.: CD004514. DOI: 10.1002/14651858.CD004514.147. Vollset SE et al. Plasma total homocysteine, pregnancy complicati<strong>on</strong>s, and adversepregnancy outcomes: the Hordaland Homocysteine study. American Journal of ClinicalNutriti<strong>on</strong>, 2000, 71:962–988.148. Ericks<strong>on</strong> JD et al. Folate status in women of childbearing age, by race/ethnicity –United States, 1999–2000. Morbidity and Mortality Weekly Report, 2002, 51:808–810.149. Lawrence JM et al. Trends in serum folate after <strong>food</strong> fortificati<strong>on</strong>. Lancet, 1999,354:915–916.150. Allen LH. Folate and vitamin B12 status in the Americas. Nutriti<strong>on</strong> Reviews, 2004,62 (6 Pt 2):S29–S33.151. Refsum H et al. Hyperhomocysteinemia and elevated methylmal<strong>on</strong>ic acid indicatea high prevalence of cobalamin deficiency in Asian Indians. American Journal ofClinical Nutriti<strong>on</strong>, 2001, 74:233–241.152. Siekmann JH et al. Kenyan school children have multiple micr<strong>on</strong>utrient deficiencies,but increased plasma vitamin B-12 is the <strong>on</strong>ly detectable micr<strong>on</strong>utrient resp<strong>on</strong>se tomeat or milk supplementati<strong>on</strong>. Journal of Nutriti<strong>on</strong>, 2003, 133:3972S–3980S.153. Krajcovicova-Kudlackova M et al. Homocysteine levels in vegetarians versus omnivores.Annals of Nutriti<strong>on</strong> & Metabolism, 2000, 44:135–138.154. Healt<strong>on</strong> EB et al. Neurologic aspects of cobalamin deficiency. Medicine (Baltimore),1991, 70:229–245.155. Allen LH et al. Cognitive and neuromotor performance of Guatemalan schoolers<strong>with</strong> deficient, marginal and normal plasma B-12. FASEB Journal, 1999, 13:A544.156. Allen LH. Impact of vitamin B-12 deficiency during lactati<strong>on</strong> <strong>on</strong> maternal andinfant health. Advances in Experimental Medicine and Biology, 2002, 503:57–67.157. Martin DC et al. Time dependency of cognitive recovery <strong>with</strong> cobalamin replacement:report of a pilot study. Journal of the American Geriatrics Society, 1992,40:168–172.158. Thiamine deficiency and its preventi<strong>on</strong> and c<strong>on</strong>trol in major emergencies. Geneva,WorldHealth Organizati<strong>on</strong>, 1999 (WHO/NHD/99.13).159. Djoenaidi W, Notermans SL, Verbeek AL. Subclinical beriberi polyneuropathy inthe low income group: an investigati<strong>on</strong> <strong>with</strong> special tools <strong>on</strong> possible patients <strong>with</strong>suspected complaints. European Journal of Clinical Nutriti<strong>on</strong>, 1996, 50:549–555.160. Bovet P et al. Blood thiamin status and determinants in the populati<strong>on</strong> of Seychelles(Indian Ocean). Journal of Epidemiology and Community Health, 1998, 52:237–242.161. Butterworth RF. Maternal thiamine deficiency: still a problem in some world communities.American Journal of Clinical Nutriti<strong>on</strong>, 2001, 74:712–713.162. McGready R et al. Postpartum thiamine deficiency in a Karen displaced populati<strong>on</strong>.American Journal of Clinical Nutriti<strong>on</strong>, 2001, 74:808–813.163. Tang CM et al. Outbreak of beri-beri in The Gambia. Lancet, 1989, 2:206–207.164. Macias-Matos C et al. Biochemical evidence of thiamine depleti<strong>on</strong> during theCuban neuropathy epidemic, 1992–1993. American Journal of Clinical Nutriti<strong>on</strong>,1996, 64:347–353.165. Bates C et al. Reply to D.A. Gans. American Journal of Clinical Nutriti<strong>on</strong>, 1997,65:1091.267
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS166. L<strong>on</strong>sdale D. Thiamine deficiency and sudden deaths. Lancet, 1990, 336:376.167. Combs GF Jr. The vitamins: fundamental aspects in nutriti<strong>on</strong> and health. 2nd ed. SanDiego, CA, Academic Press, 1992.168. Bhuvaneswaran C, Sreenivasan A. Problems of thiamine deficiency states and theirameliorati<strong>on</strong>. Annals of the New York Academy of Sciences, 1962, 98:576–601.169. Vimokesant SL et al. Effects of betel nut and fermented fish <strong>on</strong> the thiaminstatus of northeastern Thais. American Journal of Clinical Nutriti<strong>on</strong>, 1975, 28:1458–1463.170. Hustad S et al. Riboflavin, flavin m<strong>on</strong><strong>on</strong>ucleotide, and flavin adenine dinucleotidein human plasma and erythrocytes at baseline and after low-dose riboflavin supplementati<strong>on</strong>.Clinical Chemistry, 2002, 48:1571–1577.171. Graham JM et al. Riboflavin status of pregnant Nepali women; comparis<strong>on</strong> oferythrocyte riboflavin <strong>with</strong> erythrocyte reductase activity coefficient (EGRAC)methods. FASEB Journal, 2002, 16:A276-A277.172. Allen LH. Micr<strong>on</strong>utrients. In: Flores R, Gillespie S, eds. Health and nutriti<strong>on</strong>: emergingand reemerging issues in developing countries.Washingt<strong>on</strong>, DC, Internati<strong>on</strong>al FoodPolicy Research Institute, 2001: 10. (2020 Visi<strong>on</strong> Focus No. 5).173. Reddy VA et al. Riboflavin, folate and vitamin C status of Gambian women duringpregnancy: a comparis<strong>on</strong> between urban and rural communities. Transacti<strong>on</strong>s of theRoyal Society of Tropical Medicine and Hygiene, 1987, 81:1033–1037.174. Boisvert WA et al. Prevalence of riboflavin deficiency am<strong>on</strong>g Guatemalan elderlypeople and its relati<strong>on</strong>ship to milk intake. American Journal of Clinical Nutriti<strong>on</strong>,1993, 58:85–90.175. Campbell TC et al. Questi<strong>on</strong>ing riboflavin recommendati<strong>on</strong>s <strong>on</strong> the basis of asurvey in China. American Journal of Clinical Nutriti<strong>on</strong>, 1990, 51:436–445.176. Allen LH et al. Supplementati<strong>on</strong> of anemic lactating Guatemalan women <strong>with</strong>riboflavin improves erythrocyte riboflavin c<strong>on</strong>centrati<strong>on</strong>s and ferritin resp<strong>on</strong>se toir<strong>on</strong> treatment. Journal of Nutriti<strong>on</strong>. In press.177. Powers HJ et al. The relative effectiveness of ir<strong>on</strong> and ir<strong>on</strong> <strong>with</strong> riboflavin in correctinga microcytic anaemia in men and children in rural Gambia. Human Nutriti<strong>on</strong>:Clinical Nutriti<strong>on</strong>, 1983, 37:413–425.178. Pellagra and its preventi<strong>on</strong> and c<strong>on</strong>trol in major emergencies. Geneva, World HealthOrganizati<strong>on</strong>, 2000 (WHO/NHD/00.10).179. Malfait P et al. An outbreak of pellagra related to changes in dietary niacin am<strong>on</strong>gMozambican refugees in Malawi. Internati<strong>on</strong>al Journal of Epidemiology, 1993,22:504–511.180. Setiawan B, Giraud DW, Driskell JA. Vitamin B-6 inadequacy is prevalent in ruraland urban Ind<strong>on</strong>esian children. Journal of Nutriti<strong>on</strong>, 2000, 130:553–558.181. McCullough AL et al. Vitamin B-6 status of Egyptian mothers: relati<strong>on</strong> to infantbehavior and maternal-infant interacti<strong>on</strong>s. American Journal of Clinical Nutriti<strong>on</strong>,1990, 51:1067–1074.182. Fairfield KM, Fletcher RH. Vitamins for chr<strong>on</strong>ic disease preventi<strong>on</strong> in adults:scientific review. Journal of the American Medical Associati<strong>on</strong>, 2002, 287:3116–3126.183. Chang SJ, Kirksey A. Pyridoxine supplementati<strong>on</strong> of lactating mothers: relati<strong>on</strong> tomaternal nutriti<strong>on</strong> status and vitamin B-6 c<strong>on</strong>centrati<strong>on</strong>s in milk. American Journalof Clinical Nutriti<strong>on</strong>, 1990, 51:826–831.184. Scurvy and its preventi<strong>on</strong> and c<strong>on</strong>trol in major emergencies. Geneva, World HealthOrganizati<strong>on</strong>, 1999 (WHO/NHD/99.11).268
REFERENCES185. Centers for Disease C<strong>on</strong>trol and Preventi<strong>on</strong> (CDC). Nutriti<strong>on</strong> and health statusof displaced pers<strong>on</strong>s – Sudan, 1988–1989. Morbidity and Mortality Weekly Report,1989, 38:848–855.186. Desenclos JC et al. Epidemiological patterns of scurvy am<strong>on</strong>g Ethiopian refugees.Bulletin of the World Health Organizati<strong>on</strong>, 1989, 67:309–316.187. Toole MJ. Micr<strong>on</strong>utrient deficiencies in refugees. Lancet, 1992, 339:1214–1216.188. Grusin H, Kincaid-Smith PS. Scurvy in adult Africans – a clinical, haematological,and pathological study. American Journal of Clinical Nutriti<strong>on</strong>, 1954, 2:323–335.189. Hampl JS,Taylor CS, Johnst<strong>on</strong> CS. NHANES III data indicate that American subgroupshave a high risk of vitamin C deficiency. Journal of the American DieteticAssociati<strong>on</strong>, 2000, 100:A-59 (Abstract).190. Sauberlich HE, Skala OH, Dowdy RP. Laboratory tests for the assessment of nutriti<strong>on</strong>alstatus. Cleveland, OH, CRC Press, 1974.191. Severs D,Williams T, Davies JW. Infantile scurvy – a public health problem. CanadianJournal of Public Health, 1961, 52:214–220.192. Turner E, Pitt D,Thoms<strong>on</strong> R. Scurvy yesterday and today. Medical Journal of Australia,1959, 46:243–246.193. Food and Nutriti<strong>on</strong> Board, Institute of Medicine. Dietary reference intakes forcalcium, phosphorus, magnesium, vitamin D, and fluoride. Washingt<strong>on</strong>, DC, Nati<strong>on</strong>alAcademy Press, 1999.194. Specker BL et al. Prospective study of vitamin D supplementati<strong>on</strong> and rickets inChina. Journal of Pediatrics, 1992, 120:733–739.195. Zeghoud F et al. Subclinical vitamin D deficiency in ne<strong>on</strong>ates: definiti<strong>on</strong> andresp<strong>on</strong>se to vitamin D supplements. American Journal of Clinical Nutriti<strong>on</strong>, 1997,65:771–778.196. Dagnelie PC et al. High prevalence of rickets in infants <strong>on</strong> macrobiotic diets. AmericanJournal of Clinical Nutriti<strong>on</strong>, 1990, 51:202–208.197. Lebrun JB et al. Vitamin D deficiency in a Manitoba community. Canadian Journalof Public Health, 1993, 84:394–396.198. Yan L et al. Vitamin D status and parathyroid horm<strong>on</strong>e c<strong>on</strong>centrati<strong>on</strong>s in Chinesewomen and men from north-east of the People’s Republic of China. EuropeanJournal of Clinical Nutriti<strong>on</strong>, 2000, 54:68–72.199. Du X et al. Vitamin D deficiency and associated factors in adolescent girls inBeijing. American Journal of Clinical Nutriti<strong>on</strong>, 2001, 74:494–500.200. Goswami R et al. Prevalence and significance of low 25-hydroxyvitamin D c<strong>on</strong>centrati<strong>on</strong>sin healthy subjects in Delhi. American Journal of Clinical Nutriti<strong>on</strong>, 2000,72:472–475.201. El-S<strong>on</strong>baty MR, Abdul-Ghaffar NU. Vitamin D deficiency in veiled Kuwaitiwomen. European Journal of Clinical Nutriti<strong>on</strong>, 1996, 50:315–318.202. Kreiter SR et al. Nutriti<strong>on</strong>al rickets in African American breast-fed infants. Journalof Pediatrics, 2000, 137:153–157.203. Thacher TD et al. A comparis<strong>on</strong> of calcium, vitamin D, or both for nutriti<strong>on</strong>alrickets in Nigerian children. New England Journal of Medicine, 1999, 341:563–568.204. Daws<strong>on</strong>-Hughes B et al. Effect of vitamin D supplementati<strong>on</strong> <strong>on</strong> wintertime andoverall b<strong>on</strong>e loss in healthy postmenopausal women. Annals of Internal Medicine,1991, 115:505–512.205. Lee WT et al. B<strong>on</strong>e mineral c<strong>on</strong>tent of two populati<strong>on</strong>s of Chinese children <strong>with</strong>different calcium intakes. B<strong>on</strong>e and Mineral, 1993, 23:195–206.269
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS206. Dibba B et al. Effect of calcium supplementati<strong>on</strong> <strong>on</strong> b<strong>on</strong>e mineral accreti<strong>on</strong> inGambian children accustomed to a low-calcium diet. American Journal of ClinicalNutriti<strong>on</strong>, 2000, 71:544–549.207. Yang GQ et al. The role of selenium in Keshan disease. Advances in Nutriti<strong>on</strong>alResearch, 1984, 6:203–231.208. Food and Nutriti<strong>on</strong> Board, Institute of Medicine. Dietary reference Intakes forVitamin C, Vitamin E, Selenium, and Carotenoids. Washingt<strong>on</strong>, DC, Nati<strong>on</strong>alAcademy Press, 2000.209. Fox TE, Fairweather-Tait S. Selenium. In: Hurrell RF, ed. The mineral fortificati<strong>on</strong>of <strong>food</strong>s. Leatherhead, Surrey, Leatherhead Publishing, 1999: 112–153.210. Trace Elements in Human Nutriti<strong>on</strong> and Health. Geneva,World Health Organizati<strong>on</strong>,1996.211. Ge K, Yang G. The epidemiology of selenium deficiency in the etiological studyof endemic diseases in China. American Journal of Clinical Nutriti<strong>on</strong>, 1993, 57(2 Suppl):259S–263S.212. Chen XS et al. Studies <strong>on</strong> the relati<strong>on</strong>s of selenium and Keshan disease. BiologicalTrace Element Research, 1980, 2:91–107.213. Zhang WH et al. Selenium, iodine and fungal c<strong>on</strong>taminati<strong>on</strong> in Yulin District(People’s Republic of China) endemic for Kashin-Beck disease. Internati<strong>on</strong>alOrthopaedics, 2001, 25:188–190.214. Moreno-Reyes R et al. Kashin-Beck disease and iodine deficiency in Tibet. Internati<strong>on</strong>alOrthopaedics, 2001, 25:164–166.215. Vanderpas JB et al. Selenium deficiency mitigates hypothyroxinemia in iodinedeficientsubjects. American Journal of Clinical Nutriti<strong>on</strong>, 1993, 57 (2 Suppl):271S–275S.216. Giray B et al. Status of selenium and antioxidant enzymes of goitrous children islower than healthy c<strong>on</strong>trols and n<strong>on</strong>goitrous children <strong>with</strong> high iodine deficiency.Biological Trace Element Research, 2001, 82:35–52.217. Aro A, Alfthan G, Varo P. Effects of supplementati<strong>on</strong> of fertilizers <strong>on</strong> human seleniumstatus in Finland. Analyst, 1995, 120:841–843.218. Cheng YY, Qian PC. The effect of selenium-fortified table salt in the preventi<strong>on</strong> ofKeshan disease <strong>on</strong> a populati<strong>on</strong> of 1.05 milli<strong>on</strong>. Biomedical and Envir<strong>on</strong>mentalSciences, 1990, 3:422–428.219. Fluorides and oral health. Report of a WHO Expert Committee <strong>on</strong> Oral Health Statusand Fluoride Use. Geneva, World Health Organizati<strong>on</strong>, 1994 (WHO TechnicalReport Series, No. 846).220. Hillier S et al. Fluoride in drinking water and risk of hip fracture in the UK: a casec<strong>on</strong>trolstudy. Lancet, 2000, 355:265–269.221. Demos LL et al. Water fluoridati<strong>on</strong>, osteoporosis, fractures – recent developments.Australian Dental Journal, 2001, 46:80–87.222. Phipps KR et al. Community water fluoridati<strong>on</strong>, b<strong>on</strong>e mineral density, and fractures:prospective study of effects in older women. British Medical Journal, 2000,321:860–864.223. Semba RD et al. Impact of vitamin A supplementati<strong>on</strong> <strong>on</strong> hematological indicatorsof ir<strong>on</strong> metabolism and protein status in children. Nutriti<strong>on</strong> Research, 1992,12:469–478.224. Hurrell RF. How to ensure adequate ir<strong>on</strong> absorpti<strong>on</strong> from ir<strong>on</strong>-fortified <strong>food</strong>. Nutriti<strong>on</strong>Reviews, 2002, 60 (7 Pt 2):S7–S15.225. Hurrell RF. Ir<strong>on</strong>. In: Hurrell RF, ed. The mineral fortificati<strong>on</strong> of <strong>food</strong>. Leatherhead,Surrey, Leatherhead Publishing, 1999: 54–93.270
REFERENCES226. Swain JH, Newman SM, Hunt JR. Bioavailability of elemental ir<strong>on</strong> powders to ratsis less than bakery-grade ferrous sulfate and predicted by ir<strong>on</strong> solubility and particlesurface area. Journal of Nutriti<strong>on</strong>, 2003, 133:3546–3552.227. Hurrell RF et al. Ferrous fumarate fortificati<strong>on</strong> of a chocolate drink powder. BritishJournal of Nutriti<strong>on</strong>, 1991, 65:271–283.228. Theuer RC et al. Effect of processing <strong>on</strong> availability of ir<strong>on</strong> salts in liquid infantsformula products – experimental milk-based formulas. Journal of Agricultural andFood Chemistry, 1973, 21:482–485.229. Hurrell RF et al. The usefulness of elemental ir<strong>on</strong> for cereal flour fortificati<strong>on</strong>: aSUSTAIN Task Force report. Nutriti<strong>on</strong> Reviews, 2002, 60:391–406.230. Lee PW, Eisen WB, German RM, eds. Handbook of powder metal technologies andapplicati<strong>on</strong>s. Materials Park, OH, American Society of Metals, 1998.231. Internati<strong>on</strong>al Nutriti<strong>on</strong>al Anemia C<strong>on</strong>sultative Group (INACG). Ir<strong>on</strong> EDTA for <strong>food</strong>fortificati<strong>on</strong>. Washingt<strong>on</strong>, DC, Internati<strong>on</strong>al Life Sciences Institute, 1993.232. Hurrell RF et al. An evaluati<strong>on</strong> of EDTA compounds for ir<strong>on</strong> fortificati<strong>on</strong> of cerealbased<strong>food</strong>s. British Journal of Nutriti<strong>on</strong>, 2000, 84:903–910.233. Davidss<strong>on</strong> L et al. Ir<strong>on</strong> bioavailability from ir<strong>on</strong>-fortified Guatemalan meals based<strong>on</strong> corn tortillas and black bean paste. American Journal of Clinical Nutriti<strong>on</strong>, 2002,75:535–539.234. Fairweather-Tait SJ et al. Ir<strong>on</strong> absorpti<strong>on</strong> from a breakfast cereal: effects of EDTAcompounds and ascorbic acid. Internati<strong>on</strong>al Journal of Vitamin and Nutriti<strong>on</strong>Research, 2001, 71:117–122.235. Barclay D et al. Cereal products having low phytic acid c<strong>on</strong>tent. Societe des ProduitsNestlé S.A.Federal Institute of Technology Zurich. Internati<strong>on</strong>al Patent Applicati<strong>on</strong>PCT/EP00/05140, publicati<strong>on</strong> No. WO/00/72700, 2000.236. Egli I. Traditi<strong>on</strong>al <strong>food</strong> processing methods to increase mineral bioavailability from cerealand legume based weaning <strong>food</strong>s [Dissertati<strong>on</strong>]. Swiss Federal Institute of Technology,Zurich, 2001.237. Dary O, Freire W, Kim S. Ir<strong>on</strong> compounds for <strong>food</strong> fortificati<strong>on</strong>: guidelinesfor Latin America and the Caribbean 2002. Nutriti<strong>on</strong> Reviews, 2002, 60:S50–S61.238. Evaluati<strong>on</strong> of certain <strong>food</strong> additives and c<strong>on</strong>taminants. Fifty-third report of the JointFAO/WHO Expert Committee <strong>on</strong> Food Additives. Geneva, World Health Organizati<strong>on</strong>,2000 (WHO Technical Series No. 896).239. Allen LH. Advantages and limitati<strong>on</strong>s of ir<strong>on</strong> amino acid chelates as ir<strong>on</strong> fortificants.Nutriti<strong>on</strong> Reviews, 2002, 60 (Suppl 1):S18–S21.240. Bovell-Benjamin AC, Viteri FE, Allen LH. Ir<strong>on</strong> absorpti<strong>on</strong> from ferrous bisglycinateand ferric trisglycinate in whole maize is regulated by ir<strong>on</strong> status. AmericanJournal of Clinical Nutriti<strong>on</strong>, 2000, 71:1563–1569.241. Fidler MC et al. A micr<strong>on</strong>ised, dispersible ferric pyrophosphate <strong>with</strong> high relativebioavailability in man. British Journal of Nutriti<strong>on</strong>, 2004, 91:107–112.242. Zimmermann MB et al. Comparis<strong>on</strong> of the efficacy of wheat-based snacks fortified<strong>with</strong> ferrous sulfate, electrolytic ir<strong>on</strong>, or hydrogen-reduced elemental ir<strong>on</strong>: randomized,double-blind, c<strong>on</strong>trolled trial in Thai women. American Journal of ClinicalNutriti<strong>on</strong>, 2005, 82:1276–1282.243. Dary O. Less<strong>on</strong>s learned <strong>with</strong> ir<strong>on</strong> fortificati<strong>on</strong> in Central America. Nutriti<strong>on</strong>Reviews, 2002, 60 (7 Pt 2):S30–S33.244. Sarker SA et al. Helicobacter pylori infecti<strong>on</strong>, ir<strong>on</strong> absorpti<strong>on</strong>, and gastric acidsecreti<strong>on</strong> in Bangladeshi children. American Journal of Clinical Nutriti<strong>on</strong>, 2004,80:149–153.271
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS245. Wang CF, King RL. Chemical and sensory evaluati<strong>on</strong> of ir<strong>on</strong>-fortified milk. Journalof Food Science, 1973, 38:938–940.246. Moretti D et al. Development and Evaluati<strong>on</strong> of Ir<strong>on</strong>-fortified Extruded RiceGrains. Journal of Food Science, 2005, 70:S330–S336.247. Douglas FW et al. Color, flavor, and ir<strong>on</strong> bioavailability in ir<strong>on</strong>-fortified chocolatemilk. Journal of Dairy Science, 1981, 64:1785–1793.248. Davidss<strong>on</strong> L et al. Influence of ascorbic acid <strong>on</strong> ir<strong>on</strong> absorpti<strong>on</strong> from an ir<strong>on</strong>fortified,chocolate-flavored milk drink in Jamaican children. American Journal ofClinical Nutriti<strong>on</strong>, 1998, 67:873–877.249. Fidler MC et al. Ir<strong>on</strong> absorpti<strong>on</strong> from fish sauce and soy sauce fortified <strong>with</strong> sodiumir<strong>on</strong> EDTA. American Journal of Clinical Nutriti<strong>on</strong>, 2003, 78:274–278.250. Huo J et al. Therapeutic effects of NaFeEDTA-fortified soy sauce in anaemicchildren in China. Asia Pacific Journal of Clinical Nutriti<strong>on</strong>, 2002, 11:123–127.251. Oppenheimer SJ. Ir<strong>on</strong> and its relati<strong>on</strong> to immunity and infectious disease. Journalof Nutriti<strong>on</strong>, 2001, 131 (2S-2):616S–633S.252. Heresi G et al. Effect of supplementati<strong>on</strong> <strong>with</strong> an ir<strong>on</strong>-fortified milk <strong>on</strong> incidenceof diarrhea and respiratory infecti<strong>on</strong> in urban-resident infants. Scandinavian Journalof Infectious Diseases, 1995, 27:385–389.253. Hemminki E et al. Impact of ir<strong>on</strong> fortificati<strong>on</strong> of milk formulas <strong>on</strong> infants growthand health. Nutriti<strong>on</strong> Research, 1995, 15:491–503.254. Power HM et al. Ir<strong>on</strong> fortificati<strong>on</strong> of infant milk formula: the effect <strong>on</strong> ir<strong>on</strong> statusand immune functi<strong>on</strong>. Annals of Tropical Paediatrics, 1991, 11:57–66.255. Brunser O et al. Chr<strong>on</strong>ic ir<strong>on</strong> intake and diarrhoeal disease in infants. A field studyin a less-developed country. European Journal of Clinical Nutriti<strong>on</strong>, 1993, 47:317–326.256. Danesh J, Appleby P. Cor<strong>on</strong>ary heart disease and ir<strong>on</strong> status: meta-analyses ofprospective studies. Circulati<strong>on</strong>, 1999, 99:852–854.257. Lund EK et al. Oral ferrous sulfate supplements increase the free radicalgeneratingcapacity of feces from healthy volunteers. American Journal of ClinicalNutriti<strong>on</strong>, 1999, 69:250–255.258. Stevens RG et al. Body ir<strong>on</strong> stores and the risk of cancer. New England Journal ofMedicine, 1988, 319:1047–1052.259. Arya SS, Thakur BR. Effect of water activity <strong>on</strong> vitamin A degradati<strong>on</strong> in wheatflour (atta). Journal of Food Processing and Preservati<strong>on</strong>, 1990, 14:123–134.260. Favaro RMD et al. Studies <strong>on</strong> fortificati<strong>on</strong> of refined soybean oil <strong>with</strong> all-transretinylpalmitate in Brazil: stability during cooking and storage. Journal of Food Compositi<strong>on</strong>and Analysis, 1991, 4:237–244.261. Fortificati<strong>on</strong> Basics: Sugar. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrient Interventi<strong>on</strong>s,1997.262. Ols<strong>on</strong> JA. Vitamin A. In: Ziegler EE, Filer LJ, eds. Present knowledge in nutriti<strong>on</strong>.Washingt<strong>on</strong>, DC, Internati<strong>on</strong>al Life Sciences Institute Press, 1996: 109–119.263. Dary O, Mora JO. Food fortificati<strong>on</strong> to reduce vitamin A deficiency: Internati<strong>on</strong>alVitamin A C<strong>on</strong>sultative Group recommendati<strong>on</strong>s. Journal of Nutriti<strong>on</strong>, 2002, 132(9 Suppl):2927S–2933S.264. Johns<strong>on</strong> LE. Oils, fats and margarine: overview of technology. In: Micr<strong>on</strong>utrientInitiative, ed. Food fortificati<strong>on</strong> to end micr<strong>on</strong>utrient malnutriti<strong>on</strong>. State of the Art.Ottawa, Micr<strong>on</strong>utrient Initiative, 1998: 22–26.265. Bloch CE. Effects of deficiency in vitamins in infancy. American Journal of Diseasesof Children, 1931, 42:271.272
REFERENCES266. Aykroyd WR et al. Medical Resurvey of Nutriti<strong>on</strong> in Newfoundland 1948. CanadianMedical Associati<strong>on</strong> Journal, 1949, 60:329–352.267. Sridhar KK. Tackling micr<strong>on</strong>utrient malnutriti<strong>on</strong>: Two case studies in India. In:Micr<strong>on</strong>utrient Initiative, ed. Food fortificati<strong>on</strong> to end micr<strong>on</strong>utrient malnutriti<strong>on</strong>. Stateof the Art. Ottawa, Micr<strong>on</strong>utrient Initiative, 1998: 32–36.268. Atwood SJ et al. Stability of vitamin A in fortified vegetable oil and corn soy blendused in child feeding programs in India. Journal of Food Compositi<strong>on</strong> and Analysis,1995, 8:32–44.269. Opportunities for Micr<strong>on</strong>utrient Interventi<strong>on</strong>s (OMNI). Fortificati<strong>on</strong> of wheat flour<strong>with</strong> vitamin A: an update. Washingt<strong>on</strong>, D.C., US Agency for Internati<strong>on</strong>al Development,1998.270. Final Report of the Micr<strong>on</strong>utrient Assessment Project.Washingt<strong>on</strong>, DC, Sharing UnitedStates Technology to Aid in the Improvement of Nutriti<strong>on</strong>, 1999.271. Chavez JF. Enrichment of precooked corn flour and wheat flour in Venezuela: Asuccessful experience. In: Micr<strong>on</strong>utrient Initiative, ed. Food fortificati<strong>on</strong> to endmicr<strong>on</strong>utrient malnutriti<strong>on</strong>. State of the Art. Ottawa, Micr<strong>on</strong>utrient Initiative, 1998:62–65.272. Dary O. Sugar fortificati<strong>on</strong> <strong>with</strong> vitamin A: A Central American c<strong>on</strong>tributi<strong>on</strong> to thedeveloping world. In: Micr<strong>on</strong>utrient Initiative, ed. Food fortificati<strong>on</strong> to end micr<strong>on</strong>utrientmalnutriti<strong>on</strong>. State of the Art. Ottawa, Micr<strong>on</strong>utrient Initiative, 1998: 95–98.273. Arroyave G. The program of fortificati<strong>on</strong> of sugar <strong>with</strong> vitamin A in Guatemala:some factors bearing <strong>on</strong> its implementati<strong>on</strong> and maintenance. In: Scrimshaw NS,Wallerstein MT, eds. Nutriti<strong>on</strong> policy implementati<strong>on</strong>.Issues and experience. New York,Plenum Press, 1982: 75–88.274. Krause VM, Delisle H, Solom<strong>on</strong>s NW. Fortified <strong>food</strong>s c<strong>on</strong>tribute <strong>on</strong>e half of recommendedvitamin A intake in poor urban Guatemalan toddlers. Journal of Nutriti<strong>on</strong>,1998, 128:860–864.275. Dary O, Guamuch M, Nestel P. Recovery of retinol in soft-drink beverages made<strong>with</strong> fortified unrefined and refined sugar: implicati<strong>on</strong>s for nati<strong>on</strong>al fortificati<strong>on</strong>programs. Journal of Food Compositi<strong>on</strong> and Analysis, 1998, 11:212–220.276. Rosado JL et al. Development, producti<strong>on</strong>, and quality c<strong>on</strong>trol of nutriti<strong>on</strong>nal supplementsfor a nati<strong>on</strong>al supplementati<strong>on</strong> programme in Mexico. Food and Nutriti<strong>on</strong>Bulletin, 2000, 21:30–34.277. Tartanac F. Incaparina and other Incaparina-based <strong>food</strong>s: Experience of INCAP inCentral America. Food and Nutriti<strong>on</strong> Bulletin, 2000, 21:49–54.278. Lopez de Romana D. Experience <strong>with</strong> complementary feeding in the FONCODESproject. Food and Nutriti<strong>on</strong> Bulletin, 2000, 21:43–48.279. Chavasit V, T<strong>on</strong>tisirin K. Triple fortificati<strong>on</strong>: instant noodles in Thailand. In:Micr<strong>on</strong>utrient Initiative, ed. Food fortificati<strong>on</strong> to end micr<strong>on</strong>utrient malnutriti<strong>on</strong>. Stateof the Art. Ottawa, Micr<strong>on</strong>utrient Initiative, 1998: 72–76.280. Bynum D. Fortificati<strong>on</strong> of dairy products <strong>with</strong> micr<strong>on</strong>utrients to end malnutriti<strong>on</strong>.In: Micr<strong>on</strong>utrient Initiative, ed. Food fortificati<strong>on</strong> to end micr<strong>on</strong>utrient malnutriti<strong>on</strong>.State of the Art. Ottawa, Micr<strong>on</strong>utrient Initiative, 1998: 38–42.281. Allen LH, Haskell M. Estimating the potential for vitamin A toxicity in women andyoung children. Journal of Nutriti<strong>on</strong>, 2002, 132 (9 Suppl):2907S–2919S.282. Evaluati<strong>on</strong> of certain <strong>food</strong> additives and c<strong>on</strong>taminants.Thirty-seventh report of the JointFAO/WHO Expert Committee <strong>on</strong> Food Additives. Geneva, World Health Organizati<strong>on</strong>,1991 (WHO Technical Series No. 806).273
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS283. Recommended iodine levels in salt and guidelines for m<strong>on</strong>itoring their adequacy and effectiveness.Geneva, World Health Organizati<strong>on</strong>, 1996 (WHO/NUT/96.13).284. Progress towards the eliminati<strong>on</strong> of iodine deficiency disorders (IDD). Geneva, WorldHealth Organizati<strong>on</strong>, 1999 (WHO/NHD/99.4).285. Codex Alimentarius Commisi<strong>on</strong>. Codex Standard for Food Grade Salt. CODEXSTAN 150-1985, revised 1997, amended 2001. Rome, Joint FAO/WHO FoodStandards Programme, Codex Alimentarius Commisi<strong>on</strong>, 1985 (http://www.codexalimentarius.net/download/standards/3/CXS_150e.pdf, accessed 7 October2005).286. Burgi H. Iodizati<strong>on</strong> of salt and <strong>food</strong>. Technical and legal aspects. In: Delange F,Dunn JT, Glinoer D, eds. Iodine deficiency in Europe.A c<strong>on</strong>tinuing c<strong>on</strong>cern. New York,Plenum Press, 1993: 261–266.287. Mannar V, Dunn JT. Salt iodizati<strong>on</strong> for the eliminati<strong>on</strong> of iodine deficiency. TheNetherlands, Internati<strong>on</strong>al Council for C<strong>on</strong>trol of Iodine Deficiency Disorders,1995.288. Diosady LL et al. Stability of iodine in iodized salt used for correcti<strong>on</strong> of iodinedeficiencydisorders. II. Food and Nutriti<strong>on</strong> Bulletin, 1998, 19:240–250.289. The state of the world’s children. New York, United Nati<strong>on</strong>s Children’s Fund, 2003.290. Delange F, Hetzel BS.The iodine deficiency disorders. In: Hennemann G, DeGrootL, eds. The thyroid and its diseases. MA, Endocrine Educati<strong>on</strong>, Inc, 2003:(http://www.thyroidmanager.org, accessed 22 March 2005).291. Gerasimov G et al. Bread iodizati<strong>on</strong> for iodine deficient regi<strong>on</strong>s of Russia and othernewly independant states. IDD Newsletter, 1997, 13:12–13.292. Suwanik R, Pleehachinda R, Pattanachak C, et al. Simple technology provides effectiveIDD c<strong>on</strong>trol at the village level in Thailand. IDD Newsletter, 1989, 5:1–6.293. Fisch A et al. A new approach to combatting iodine deficiency in developing countries:the c<strong>on</strong>trolled release of iodine in water by a silic<strong>on</strong>e elastomer. AmericanJournal of Public Health, 1993, 83:540–545.294. Elnagar B et al. C<strong>on</strong>trol of iodine deficiency using iodinati<strong>on</strong> of water in a goitreendemic area. Internati<strong>on</strong>al Journal of Food Sciences and Nutriti<strong>on</strong>, 1997, 48:119–127.295. Foo LC et al. Iodizati<strong>on</strong> of village water supply in the c<strong>on</strong>trol of endemic iodinedeficiency in rural Sarawak, Malaysia. Biomedical and Envir<strong>on</strong>mental Sciences, 1996,9:236–241.296. An<strong>on</strong>ymous. Iodized water to eliminate iodine deficiency. IDD Newsletter, 1997,13:33–39.297. Cao XY et al. Iodinati<strong>on</strong> of irrigati<strong>on</strong> water as a method of supplying iodine to aseverely iodine-deficient populati<strong>on</strong> in Xinjiang, China. Lancet, 1994, 344:107–110.298. Phillips DI. Iodine, milk, and the eliminati<strong>on</strong> of endemic goiter in Britain: the storyof an accidental public health triumph. Journal of Epidemiology and CommunityHealth, 1997, 51:391–393.299. Eltom M et al. The use of sugar as a vehicle for iodine fortificati<strong>on</strong> in endemiciodine deficiency. Internati<strong>on</strong>al Journal of Food Sciences and Nutriti<strong>on</strong>, 1995, 46:281–289.300. Sinawat S. Fish sauce fortificati<strong>on</strong> in Thailand. In: Micr<strong>on</strong>utrient Initiative, ed. Foodfortificati<strong>on</strong> to end micr<strong>on</strong>utrient malnutriti<strong>on</strong>. State of the Art. Ottawa, Micr<strong>on</strong>utrientInitiative, 1998: 102–104.301. Burgi H, Schaffner TH, Seiler JP. The toxicology of iodate: a review of the literature.Thyroid, 2001, 11:449–456.274
REFERENCES302. Stanbury JB et al. Iodine-induced hyperthyroidism: occurrence and epidemiology.Thyroid, 1998, 8:83–100.303. Bourdoux PP et al. Iodine induced thyrotoxicosis in Kivu, Zaire. Lancet, 1996,347:552–553.304. Todd CH et al. Increase in thyrotoxicosis associated <strong>with</strong> iodine supplements inZimbabwe. Lancet, 1995, 346:1563–1564.305. Delange F, de Benoist B, Alnwick D. Risks of iodine-induced hyperthyroidismafter correcti<strong>on</strong> of iodine deficiency by iodized salt. Thyroid, 1999, 9:545–556.306. Todd CH. Hyperthyroidism and other thyroid disorders: a practical handbook for recogniti<strong>on</strong>and management. Geneva, World Health Organizati<strong>on</strong>, 1999 (WHO/AFRO/NUT/99.1).307. Laurberg P et al. Thyroid disorders in mild iodine deficiency. Thyroid, 2000,10:951–963.308. Diaz M et al. Bioavailability of zinc sulfate and zinc oxide added to corn tortilla. Astudy using stable isotopes. FASEB Journal, 2001, 15:A578.5 (Abstract).309. Lopez de Romana D, L<strong>on</strong>nerdal B, Brown KH. Absorpti<strong>on</strong> of zinc from wheatproducts fortified <strong>with</strong> ir<strong>on</strong> and either zinc sulfate or zinc oxide. American Journalof Clinical Nutriti<strong>on</strong>, 2003, 78:279–283.310. Hurrell RF et al. Degradati<strong>on</strong> of phytic acid in cereal porridges improves ir<strong>on</strong>absorpti<strong>on</strong> by human subjects. American Journal of Clinical Nutriti<strong>on</strong>, 2003,77:1213–1219.311. Davidss<strong>on</strong> L, Kastenmayer P, Hurrell RF. Sodium ir<strong>on</strong> EDTA [NaFe(III)EDTA]as a <strong>food</strong> fortificant: the effect <strong>on</strong> the absorpti<strong>on</strong> and retenti<strong>on</strong> of zinc and calciumin women. American Journal of Clinical Nutriti<strong>on</strong>, 1994, 60:231–237.312. Hambidge KM et al. Zinc nutriti<strong>on</strong>al status of young middle-income children andeffects of c<strong>on</strong>suming zinc-fortified breakfast cereals. American Journal of ClinicalNutriti<strong>on</strong>, 1979, 32:2532–2539.313. Kilic I et al. The effect of zinc-supplemented bread c<strong>on</strong>sumpti<strong>on</strong> <strong>on</strong> school children<strong>with</strong> asymptomatic zinc deficiency. Journal of Pediatric Gastroenterology andNutriti<strong>on</strong>, 1998, 26:167–171.314. Lopez de Romana D, Brown KH, Guinard JX. Sensory trial to assess the acceptabilityof zinc fortificants added to ir<strong>on</strong>-fortified wheat products. Journal of FoodScience, 2002, 67:461–465.315. Pfeiffer CM et al. Absorpti<strong>on</strong> of folate from fortified cereal-grain products andof supplemental folate c<strong>on</strong>sumed <strong>with</strong> or <strong>with</strong>out <strong>food</strong> determined by using adual-label stable-isotope protocol. American Journal of Clinical Nutriti<strong>on</strong>, 1997,66:1388–1397.316. Fortificati<strong>on</strong> Basics: Stability. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrient Interventi<strong>on</strong>s,1998.317. Bauernfeind JC, DeRitter E. Foods c<strong>on</strong>sidered for nutrient additi<strong>on</strong>: cereal grainproducts. In: Bauernfeind JC, Lachance PA, eds. Nutrient additi<strong>on</strong>s to <strong>food</strong>: nutriti<strong>on</strong>al,technological and regulatory aspects. Trumbull, CT, Food and Nutriti<strong>on</strong> Press,1991: 143–209.318. Bowley A, ed. Mandatory <strong>food</strong> enrichment. Basel, Roche Vitamins Europe Ltd, 2003(Nutriview Special Issue 1–12).319. Opini<strong>on</strong> of the Scientific Committee <strong>on</strong> Food <strong>on</strong> the tolerable upper intake levels ofnicotinic acid and nicotinamide (niacin). Brussels, European Commissi<strong>on</strong>, 2002(SCF/CS/NUT/UPPLEV/39).275
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS320. Flynn A, Cashman K. Calcium. In: Hurrell RF, ed. The mineral fortificati<strong>on</strong> of <strong>food</strong>s.Leatherhead, Surrey, Leatherhead Publishing, 1999: 18–53.321. Ranhotra GS, Lee C, Gelroth JA. Expanded cereal fortificati<strong>on</strong> – bioavailability andfuncti<strong>on</strong>ality (breadmaking) of various calcium sources. Nutriti<strong>on</strong> Reports Internati<strong>on</strong>al,1980, 22:469–475.322. Van Dael P et al. Comparis<strong>on</strong> of selenite and selenate apparent absorpti<strong>on</strong> andretenti<strong>on</strong> in infants using stable isotope methodology. Pediatric Research, 2002,51:71–75.323. Estupinan-Day SR et al. Salt fluoridati<strong>on</strong> and dental caries in Jamaica. CommunityDentistry and Oral Epidemiology, 2001, 29:247–252.324. Stephen KW et al. Effect of fluoridated salt intake in infancy: a blind caries andfluorosis study in 8th grade Hungarian pupils. Community Dentistry and Oral Epidemiology,1999, 27:210–215.325. Nati<strong>on</strong>al Program of Salt Fluoridati<strong>on</strong>. Salt fluoridati<strong>on</strong> program in Costa Rica. TresRios, Instituto Costarricense de Investigación y Enseñanza en Nutrición y Salud(INCIENSA), 2002.326. Stephen KW, Banoczy J, Pakhomov GN, eds. Milk fluoridati<strong>on</strong> for the preventi<strong>on</strong>of dental caries. Geneva, World Health Organizati<strong>on</strong>, 1996 (WHO/ORH/MF/DOC96.1).327. Woodward SM et al. School milk as a vehicle for fluoride in the United Kingdom.An interim report. Community Dental Health, 2001, 18:150–156.328. Marino R, Villa A, Guerrero S. A community trial of fluoridated powdered milk inChile. Community Dentistry and Oral Epidemiology, 2001, 29:435–442.329. Bian JY et al. Effect of fluoridated milk <strong>on</strong> caries in primary teeth: 21-m<strong>on</strong>th results.Community Dentistry and Oral Epidemiology, 2003, 31:241–245.330. Stephen KW et al. Five-year double-blind fluoridated milk study in Scotland. CommunityDentistry and Oral Epidemiology, 1984, 12:223–229.331. Ketley CE, West JL, Lenn<strong>on</strong> MA. The use of school milk as a vehicle for fluoridein Knowsley, UK; an evaluati<strong>on</strong> of effectiveness. Community Dental Health, 2003,20:83–88.332. Food and Nutriti<strong>on</strong> Board, Institute of Medicine. Dietary reference intakes: applicati<strong>on</strong>sin dietary planning. Washingt<strong>on</strong>, DC, Nati<strong>on</strong>al Academy Press, 2003.333. Food and Nutriti<strong>on</strong> Board, Institute of Medicine. Dietary reference intakes: applicati<strong>on</strong>sin dietary assessment. Washingt<strong>on</strong>, DC, Nati<strong>on</strong>al Academy Press, 2000.334. Department of Health. Dietary Reference Values of <strong>food</strong> energy and nutrients for theUnited Kingdom. L<strong>on</strong>d<strong>on</strong>, Her Majesty’s Stati<strong>on</strong>ery Office, 1991.335. Scientific Committee for Food. Nutrient and energy intakes for the European Community.Reports of the Scientific Committee for Food. Luxembourg, Commissi<strong>on</strong> ofthe European Community, 1992 (31st Series).336. Nusser SM et al. A semiparametric transformati<strong>on</strong> approach to estimating usualdaily intake distributi<strong>on</strong>s. Journal of the American Statistical Associati<strong>on</strong>, 1996,91:1440–1449.337. Guenther PM, Kott PS, Carriquiry AL. Development of an approach for estimatingusual nutrient intake distributi<strong>on</strong>s at the populati<strong>on</strong> level. Journal of Nutriti<strong>on</strong>,1997, 127:1106–1112.338. Nyambose J, Koski KG, Tucker KL. High intra/interindividual variance ratios forenergy and nutrient intakes of pregnant women in rural Malawi show that manydays are required to estimate usual intake. Journal of Nutriti<strong>on</strong>, 2002, 132:1313–1318.276
REFERENCES339. Technical c<strong>on</strong>sultati<strong>on</strong> <strong>on</strong> recommended levels of folic acid and vitamin B12 fortificati<strong>on</strong>in the Americas. Washingt<strong>on</strong>, DC, Pan American Health Organizati<strong>on</strong>,2003.340. Complementary feeding of young children in developing countries: a review of currentscientific knowledge. Geneva, World Health Organizati<strong>on</strong>, 1998 (WHO/NUT/98.1).341. Codex Alimentarius Commissi<strong>on</strong>. <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Formulated SupplementaryFoods for Older Infants and Young Children CAC/GL 08-1991. Joint FAO/WHOFood Standards Programme, Codex Alimentarius Commisi<strong>on</strong>, 1991 (http://www.codexalimentarius.net/download/standards/298/CXG_008e.pdf, accessed 7October 2005).342. Codex Alimentarius Commissi<strong>on</strong>. Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong> Labelling CAC/GL02-1985, (revised 1993). Joint FAO/WHO Food Standard Programme, CodexAlimentarius Commisi<strong>on</strong>, 1985 (http://www.codexalimentarius.net/download/standards/34/CXG_002e.pdf, accessed 7 October 2005).343. Codex Alimentarius Commissi<strong>on</strong>. <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong> Claims CAC/GL23-1997, (revised 2004). Joint FAO/WHO Food Standards Programme, CodexAlimentarius Commisi<strong>on</strong>, 1997 (http://www.codexalimentarius.net/download/standards/351/CXG_023e.pdf, accessed 7 October 2005).344. Habicht JP, Victora CG, Vaughan JP. Evaluati<strong>on</strong> designs for adequacy, plausibilityand probability of public health programme performance and impact. Internati<strong>on</strong>alJournal of Epidemiology, 1999, 28:10–18.345. Codex Alimentarius Commissi<strong>on</strong>. Codex Alimentarius, Volume 13 – Methods ofanalysis and sampling. 2nd ed. Rome, Joint FAO/WHO Food Standard Programme,Codex Alimentarius Commisi<strong>on</strong>, 1994.346. Nestel P, Nalubola R, Mayfield E, eds. Quality assurance as applied to micr<strong>on</strong>utrientfortificati<strong>on</strong>: guidelines for technicians, supervisors and workers, c<strong>on</strong>cerned <strong>with</strong> nutriti<strong>on</strong>.Washingt<strong>on</strong>, DC, Internati<strong>on</strong>al Life Sciences Institute Press, 2002.347. Pandav CS et al. Validati<strong>on</strong> of spot-testing kits to determine iodine c<strong>on</strong>tent in salt.Bulletin of the World Health Organizati<strong>on</strong>, 2000, 78:975–980.348. Sullivan KM, May S, Maberly G. Urinary iodine assessment: a manual <strong>on</strong> survey andlaboratory methods. Atlanta, GA, Program Against Micr<strong>on</strong>utrient Malnutriti<strong>on</strong>,2000 (2nd ed.).349. Sullivan KM et al., eds. M<strong>on</strong>itoring universal salt iodizati<strong>on</strong> programs. Atlanta, GA,Program Against Micr<strong>on</strong>utrient Malnutriti<strong>on</strong>, 1995.350. Valadez JJ. Assessing child survival programs in developing countries: testing lot qualityassurance sampling. Bost<strong>on</strong>, MA, Harvard University Press, 1991.351. Binkin N et al. Rapid nutriti<strong>on</strong> surveys – how many clusters are enough? Disasters,1992, 16:97–103.352. Valadez JJ et al. Using lot quality assurance sampling to assess measurements forgrowth m<strong>on</strong>itoring in a developing country’s primary health care system. Internati<strong>on</strong>alJournal of Epidemiology, 1996, 25:381–387.353. Valadez JJ et al. A trainers guide for baseline surveys and regular m<strong>on</strong>itoring: usingLQAS for assessing field programs in community health in developing countries.Washingt<strong>on</strong>,DC, NGO Networks for Health, 2001.354. World Bank. World Development Report 1993: Investing in health. New York, OxfordUniversity Press, 1993.355. Murray CJL, Lopez AD, eds. The global burden of disease: a comprehensive assessmentof mortality and disability from diseases, injuries, and risk factors in 1990 and projectedto 2020. Cambridge, MA, Harvard University Press, 1996.277
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS356. Nestel P, Nalubola R. Manual for wheat flour fortificati<strong>on</strong> <strong>with</strong> ir<strong>on</strong>. Part 1: <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>for the development, implementati<strong>on</strong>, m<strong>on</strong>itoring, and evaluati<strong>on</strong> of a program for wheatflour fortificati<strong>on</strong> <strong>with</strong> ir<strong>on</strong>. Arlingt<strong>on</strong>,VA, Micr<strong>on</strong>utrient Operati<strong>on</strong>al Strategies andTechnologies, United States Agency for Internati<strong>on</strong>al Development, 2000.357. Levin HM et al. Micr<strong>on</strong>utrient deficiency disorders. In: Jamis<strong>on</strong> DT et al., eds.Disease c<strong>on</strong>trol priorities in developing countries. New York, Oxford University Press,1993: 421–451.358. Populati<strong>on</strong> Health and Nutriti<strong>on</strong> Department. Bangladesh: <strong>food</strong> and nutriti<strong>on</strong> sectorreview missi<strong>on</strong>: cost-effectiveness of <strong>food</strong> and nutriti<strong>on</strong> interventi<strong>on</strong> programs. Washingt<strong>on</strong>,DC, World Bank, 1985 (No. 4974-BD).359. Mas<strong>on</strong> JB et al. The Micr<strong>on</strong>utrient Report. Current progress and trends in the c<strong>on</strong>trolof vitamin A, iodine, and ir<strong>on</strong> deficiencies. Ottawa, Micr<strong>on</strong>utrient Initiative, 2001.360. Gillespie S. Major issues in the c<strong>on</strong>trol of ir<strong>on</strong> deficiency. Ottawa, The Micr<strong>on</strong>utrientInitiative, 1998.361. Hort<strong>on</strong> S, Ross J. The ec<strong>on</strong>omics of ir<strong>on</strong> deficiency. Food Policy, 2003, 28:51–75.362. Ross JS. Relative risk of child mortality due to vitamin A deficiency. PROFILES 3Working Series No. 2. Washingt<strong>on</strong>, DC, Academy for Educati<strong>on</strong> Development, 1995(PROFILES 3 Working Series, No.2).363. Clugst<strong>on</strong> GA et al. Iodine deficiency disorders in South East Asia. In: Hetzel BS,Dunn JT, Stanbury JB, eds. The preventi<strong>on</strong> and c<strong>on</strong>trol of iodine deficiency disorders.Amsterdam, Elsevier, 1987: 273–308.364. Stoltzfus RJ, Mullany L, Black RE. Ir<strong>on</strong> deficiency anaemia. Comparative quantificati<strong>on</strong>of health risks: the global and regi<strong>on</strong>al burden of disease due to 25 selected majorrisk factors. Cambridge, Harvard University Press (in press), 2004.365. Ross J, Hort<strong>on</strong> S. Ec<strong>on</strong>omic c<strong>on</strong>sequences of ir<strong>on</strong> deficiency. Ottawa, Micr<strong>on</strong>utrientInitiative, 1998.366. Yearbook of labour statistics. 55th ed. Geneva, Internati<strong>on</strong>al Labour Organizati<strong>on</strong>,1997.367. Arkin E, Maibach E, Parvanta C. General public: communicating to persuade. In:Nels<strong>on</strong> DE et al., eds. Communicating public health informati<strong>on</strong> effectively: a guide forpractiti<strong>on</strong>ers.Washingt<strong>on</strong>, DC, American Public Health Associati<strong>on</strong>, 2002: 59–72.368. Cotento I. Nutriti<strong>on</strong> educati<strong>on</strong>: definiti<strong>on</strong>s. Journal of Nutriti<strong>on</strong> Educati<strong>on</strong>, 1995,27:279.369. Roper WL. Health communicati<strong>on</strong> takes <strong>on</strong> new dimensi<strong>on</strong>s at CDC. Public HealthReports, 1993, 108:179–183.370. Lefebvre RC, Flora JA. Social marketing and public health interventi<strong>on</strong>. HealthEducati<strong>on</strong> Quarterly, 1988, 15:299–315.371. Jernigan DH, Wright PA. Media advocacy: less<strong>on</strong>s from community experiences.Journal of Public Health Policy, 1996, 17:306–330.372. Man<strong>on</strong>court E. Participati<strong>on</strong> and social mobilizati<strong>on</strong>. Promoti<strong>on</strong> & Educati<strong>on</strong>, 1996,3:3–4, 44.373. Rothschild ML. Carrots, sticks, and promises: A c<strong>on</strong>ceptual framework for themanagement of public health and social issue behaviors. Journal of Marketing, 1999,63:24–37.374. Parvanta C. Health and nutriti<strong>on</strong> communicati<strong>on</strong>. Public Health Reviews, 2000,28:197–208.375. Browns<strong>on</strong> RC, Mal<strong>on</strong>e BR. Communicating public health informati<strong>on</strong> to policymakers. In: Nels<strong>on</strong> DE et al., eds. Communicating public health effectively: a guide forpractiti<strong>on</strong>ers.Washingt<strong>on</strong>, DC, American Public Health Associati<strong>on</strong>, 2002: 97–114.278
REFERENCES376. Smitasiri S et al. Social marketing vitamin A-rich <strong>food</strong>s in Thailand. Nakh<strong>on</strong> Pathom,Institute of Nutriti<strong>on</strong>, Mahidol University, 1993.377. Centers for Disease C<strong>on</strong>trol and Preventi<strong>on</strong> (CDC). CDCynergy 2001. Micr<strong>on</strong>utrientsediti<strong>on</strong>.Your guide to effective health communicati<strong>on</strong>s. Atlanta, GA, UnitedState Department of Health and Human Services, 2001 (http://www.cdc.gov/nccdphp/ dnpa/impact/tools/cdcynergy.htm, accessed 15 October 2005).378. Alcalay R, Bell RA. Promoting nutriti<strong>on</strong> and physical activity through social marketing:current practices and recommendati<strong>on</strong>s. Davis, CA, Center for Advanced Studiesin Nutriti<strong>on</strong> and Social Marketing, University of California, 2000.379. Saadé C, Tucker H. Bey<strong>on</strong>d pharmacies: new perspectives in ORS marketing. Arlingt<strong>on</strong>,VA, PRITECH Project, Management Sciences for Health, 1992.380. Slater S, Saadé C. Mobilizing the commercial sector for public health objectives: a practicalguide. Washingt<strong>on</strong>, DC, Basic Support for Instituti<strong>on</strong>alizing Child Survival(BASICS), 1996.381. The results of the Uruguay Round of multilateral trade negotiati<strong>on</strong>s – the legal texts.Geneva, World Trade Organizati<strong>on</strong>, 1995 (http://www.wto.org/english/docs_e/legal_e/legal_e.htm, accessed 19 April 2005).382. Codex Alimentarius Commissi<strong>on</strong>. Procedural manual. 12th ed. Rome, Food andAgriculture Organizati<strong>on</strong> of the United Nati<strong>on</strong>s, 2001.383. Codex Alimentarius Commissi<strong>on</strong>. Codex General Standard for the Labellingof Prepackaged Foods CODEX STAN 1-1985 (revised 1985, 1991, 1999, 2001).Rome, Joint FAO/WHO Food Standard Programme, Codex AlimentariusCommisi<strong>on</strong>, 1985 (CODEX STAN 01-1985, amended 2001) (http://www.codexalimentarius.net/download/standards/32/CXS_001e.pdf, accessed 7thOctober 2005).384. WTO agreements and public health: a joint study by the WHO and WTO secretariat.Geneva, World Health Organizati<strong>on</strong>, 2002.385. General Principles for the Additi<strong>on</strong> of Essential Nutrients to Foods CAC/GL 09-1987(amended 1989, 1991). Rome, Joint FAO/WHO Food Standards Programme, CodexAlimentarius Commisi<strong>on</strong>, 1987 (http://www.codexalimentarius.net/download/standards/299/CXG_009e.pdf, accessed 7 October 2005).386. Diet, nutriti<strong>on</strong> and the preventi<strong>on</strong> of chr<strong>on</strong>ic diseases. Report of Joint WHO/FAO ExpertC<strong>on</strong>sultati<strong>on</strong>. Geneva, World Health Organizati<strong>on</strong>, 2003 (WHO Technical ReportSeries No. 916).387. Institute of Medicine. Food Chemicals Codex. 5th ed. Washingt<strong>on</strong>, DC, Nati<strong>on</strong>alAcademy Press, 2003.388. British Pharmacopoeial Commissi<strong>on</strong>. The British Pharmacopoeia 2003. L<strong>on</strong>d<strong>on</strong>,Her Majesty’s Stati<strong>on</strong>ery Office, 2003.279
Further readingPart I. The role of <strong>food</strong> fortificati<strong>on</strong> in the c<strong>on</strong>trol ofmicr<strong>on</strong>utrient malnutriti<strong>on</strong> (Chapters 1 and 2)Dexter PB. Rice fortificati<strong>on</strong> for developing countries. Arlingt<strong>on</strong>, VA, Opportunitiesfor Micr<strong>on</strong>utrient Interventi<strong>on</strong>s, 1998 (No. 15) (http://www. mostproject.org/PDF/rice4.pdf,accessed 7 October 2005).Lofti M et al. Micr<strong>on</strong>utrient fortificati<strong>on</strong> of <strong>food</strong>s: current practices, research andopportunities. Ottawa, Micr<strong>on</strong>utrient Initiative, Internati<strong>on</strong>al Agricultural Centre,1996.Micr<strong>on</strong>utrient Initiative. Food fortificati<strong>on</strong> to end micr<strong>on</strong>utrient malnutriti<strong>on</strong>: Stateof-the-ArtSymposium Report, 2 August 1997, M<strong>on</strong>treal, Canada. Ottawa,Micr<strong>on</strong>utrient Initiative, Internati<strong>on</strong>al Agricultural Centre, 1998.Part II. Evaluating the public health significance ofmicr<strong>on</strong>utrient malnutriti<strong>on</strong> (Chapters 3 and 4)Sommer A. Vitamin A deficiency and its c<strong>on</strong>sequences : a field guide to detecti<strong>on</strong> andc<strong>on</strong>trol. 3rd ed. Geneva, World Health Organizati<strong>on</strong>, 1995.Part III. Fortificants: physical characteristics, selecti<strong>on</strong> anduse <strong>with</strong> specific <strong>food</strong> vehicles (Chapters 5 and 6)Arroyave G and Dary O. Manual for Sugar Fortificati<strong>on</strong> <strong>with</strong> Vitamin A. Part 1:Technical and operati<strong>on</strong>al guidelines for preparing vitamin A premix and fortifiedsugar. Arlingt<strong>on</strong>,VA, Opportunities for Micr<strong>on</strong>utrient Interventi<strong>on</strong>s, 1996 (2nd)(http://www.mostproject.org/PDF/1final.pdf, accessed 7 October 2005).Hurrell RF, ed. The mineral fortificati<strong>on</strong> of <strong>food</strong>s. Leatherhead, Surrey, LeatherheadPublishings, 1999.Fortificati<strong>on</strong> basics: milk. Arlingt<strong>on</strong>,VA, Micr<strong>on</strong>utrient Operati<strong>on</strong>al Strategies andTechnologies, The United States Agency for Internati<strong>on</strong>al DevelopementMicr<strong>on</strong>utrient Program, 1999 (http://www.mostproject.org/Updates_Feb05/Milk.pdf accessed 7 October 2005).280
FURTHER READINGFortificati<strong>on</strong> basics: maize flour/ meal. Arlingt<strong>on</strong>, VA, Micr<strong>on</strong>utrient Operati<strong>on</strong>alStrategies and Technologies, The United States Agency for Internati<strong>on</strong>al DevelopementMicr<strong>on</strong>utrient Program,1999 (http://www.mostproject.org/Updates_Feb05/Maize_Corn.pdf, accessed 7 October 2005).Fortificati<strong>on</strong> Basics: instant noodles. Arlingt<strong>on</strong>, VA, Micr<strong>on</strong>utrient Operati<strong>on</strong>alStrategies and Technologies, The United States Agency for Internati<strong>on</strong>alDevelopement Micr<strong>on</strong>utrient Program,1999 (http://www.mostproject.org/Updates_Feb05/noodles.pdf, accessed 7 October 2005).Mora JO et al. Vitamin A Sugar Fortificati<strong>on</strong> in Central America: Experience andLess<strong>on</strong>s Learned. Arlingt<strong>on</strong>, VA, Micr<strong>on</strong>utrient Operati<strong>on</strong>al Strategies and Technologies,The United States Agency for Internati<strong>on</strong>al Developement Micr<strong>on</strong>utrientProgram, 2000 (http://www.mostproject.org/PDF/sugarless<strong>on</strong>sEnglish.pdf,accessed 7 October 2005).Nalubola R and Nestel P. Wheat flour fortificati<strong>on</strong> <strong>with</strong> vitamin A. Arlingt<strong>on</strong>, VA,Opportunities for Micr<strong>on</strong>utrient Interventi<strong>on</strong>s, 1998.Manual for Wheat Flour Fortificati<strong>on</strong> <strong>with</strong> Ir<strong>on</strong>. Part 2: Technical and operati<strong>on</strong>alguidelines. Arlingt<strong>on</strong>, VA, Micr<strong>on</strong>utrient Operati<strong>on</strong>al Strategies and Technologies,The United States Agency for Internati<strong>on</strong>al Developement Micr<strong>on</strong>utrientProgram, 2000 (http://www.mostproject.org/PDF/2.pdf, accessed 7 October2005).Fortificati<strong>on</strong> basics:Wheat flour. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrientInterventi<strong>on</strong>s, 1997 (http://www.mostproject.org/Updates_Feb05/Wheat.pdf,accessed 7 October 2005).Fortificati<strong>on</strong> Basics: sugar. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrientInterventi<strong>on</strong>s, 1997 (http://www.mostproject.org/Updates_Feb05/Sugar.pdf,accessed 7 October 2005).Fortificati<strong>on</strong> basics: Oils and margarine. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrientInterventi<strong>on</strong>s, 1997 (http://www.mostproject.org/Updates_Feb05/Oils.pdf, accessed 7 October 2005).Fortificati<strong>on</strong> Basics: choosing a vehicle. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrientInterventi<strong>on</strong>s, 1997 (http://www.mostproject.org/Updates_Feb05/Vehicles.pdf, accessed 7 October 2005).Fortificati<strong>on</strong> Basics: stability. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrientInterventi<strong>on</strong>s, 1998 (http://www.mostproject.org/Updates_Feb05/Stability.pdf,accessed 7 October 2005).281
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSPart IV.Implementing effective and sustainable <strong>food</strong>fortificati<strong>on</strong> programmes (Chapters 7–11)M<strong>on</strong>itoring and evaluati<strong>on</strong> (Chapter 8)Dary O, Arroyave G. Manual for Sugar Fortificati<strong>on</strong> <strong>with</strong> Vitamin A. Part 2: <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g>for the development, implementati<strong>on</strong>, m<strong>on</strong>itoring and evaluati<strong>on</strong> of a vitamin Asugar fortificati<strong>on</strong> program. 2nd ed. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrientInterventi<strong>on</strong>s, 1996 (http://www.mostproject.org/PDF/2final.pdf, accessed7 October 2005).Dary O et al. Manual for Sugar Fortificati<strong>on</strong> <strong>with</strong> Vitamin A. Part 3: Analyticalmethods for the c<strong>on</strong>trol and evaluati<strong>on</strong> of sugar fortificati<strong>on</strong> <strong>with</strong> vitamin A.2nd ed. Arlingt<strong>on</strong>, VA, Opportunities for Micr<strong>on</strong>utrient Interventi<strong>on</strong>s, 1996(http://www.mostproject.org/PDF/3final.pdf, accessed 7 October 2005).Nalubola R, Nestel P. Manual for Wheat Flour Fortificati<strong>on</strong> <strong>with</strong> Ir<strong>on</strong>. Part 3:Analyticalmethods for m<strong>on</strong>itoring wheat flour fortificati<strong>on</strong> <strong>with</strong> ir<strong>on</strong>. Arlingt<strong>on</strong>,Virginia,Micr<strong>on</strong>utrient Operati<strong>on</strong>al Strategies and Technologies, The United StatesAgency for Internati<strong>on</strong>al Developement Micr<strong>on</strong>utrient Program, 2000(http://www.mostproject.org/PDF/3.pdf, accessed 7 October 2005).Nestel P, Nalubola R, Mayfield E. Quality assurance as applied to micr<strong>on</strong>utrientfortificati<strong>on</strong>. Washingt<strong>on</strong>, DC, Internati<strong>on</strong>al Life Sciences Institute Press, 2002(http://www.ilsi.org/file/QAtext.pdf, accessed 7 October 2005).Fortificati<strong>on</strong> basics: principles of assay procedures. Arlingt<strong>on</strong>, VA, Opportunities forMicr<strong>on</strong>utrient Interventi<strong>on</strong>s, 1998 (http://www.mostproject.org/Updates_Feb05/Assay.pdf, accessed 7 October 2005).Nati<strong>on</strong>al <strong>food</strong> law (Chapter 11)Bauernfeind JC, Lachance PA. Nutrient additi<strong>on</strong>s to <strong>food</strong>. Nutriti<strong>on</strong>al, technologicaland regulatory aspects.Trumbull, CT, Food and Nutriti<strong>on</strong> Press Inc., 1991.Nathan R. Regulati<strong>on</strong> of fortified <strong>food</strong>s to address micr<strong>on</strong>utrient malnutriti<strong>on</strong>: legislati<strong>on</strong>,regulati<strong>on</strong>s, and enforcement. Ottawa, Micr<strong>on</strong>utrient Initiative, 1999(http://www.micr<strong>on</strong>utrient.org/idpas/pdf/315Regulati<strong>on</strong>OfFortified.pdf,accessed 7 October 2005).282
A N N E X E S
ANNEX AIndicators for assessing progress towardsthe sustainable eliminati<strong>on</strong> of iodinedeficiency disordersThe internati<strong>on</strong>al community has endorsed the goal of the sustainable eliminati<strong>on</strong>of iodine deficiency as a public health problem. In order to measureprogress made towards this goal, various indicators have been developed (1).These indicators can be c<strong>on</strong>veniently grouped into three categories, namely indicatorsrelated to salt iodizati<strong>on</strong> itself, those that reflect the populati<strong>on</strong>’s iodinestatus and thirdly, those that provide a measure of the sustainability of the saltiodizati<strong>on</strong> programme. Success criteria for each of these sets of indicators havealso been established; these can to be used to assess whether the sustainable eliminati<strong>on</strong>of iodine deficiency as a public health problem has been achieved (seeTable A.1).TABLE A.1Indicators for m<strong>on</strong>itoring progress towards the sustainable eliminati<strong>on</strong> ofiodine deficiency as a public health problemIndicatorSuccess criteria/goalsSalt iodizati<strong>on</strong>Proporti<strong>on</strong> of households using adequately iodized salt a >90%Urinary iodine bProporti<strong>on</strong> of the populati<strong>on</strong> having urinary iodine below 100 µg/l
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE A.1 C<strong>on</strong>tinuedIndicatorSuccess criteria/goalsProgramme of public educati<strong>on</strong> and social mobilizati<strong>on</strong> <strong>on</strong> theimportance of iodine deficiency disorders and the c<strong>on</strong>sumpti<strong>on</strong>of iodized salt.Regular m<strong>on</strong>itoring of salt iodine at the factory, retail andhousehold levels.Regular m<strong>on</strong>itoring of urinary iodine in school-aged children, <strong>with</strong>appropriate sampling for higher risk areas.Cooperati<strong>on</strong> from the salt industry in maintenance of quality c<strong>on</strong>trol.A system for the recording of results or regular m<strong>on</strong>itoringprocedures, particularly for salt iodine, urinary iodine and, ifavailable, ne<strong>on</strong>atal thyroid stimulating horm<strong>on</strong>e, <strong>with</strong>mandatory public reporting.aAdequately iodized salt is salt that c<strong>on</strong>tains at least 15 ppm iodine. Additi<strong>on</strong>al c<strong>on</strong>diti<strong>on</strong>s forthe use of salt as a vehicle for eliminating iodine deficiency disorders are:• Local producti<strong>on</strong> and/or importati<strong>on</strong> of iodized salt in a quantity that is sufficient to satisfythe potential human demand (about 4–5 kg per pers<strong>on</strong> per year).• At the point of producti<strong>on</strong> (or importati<strong>on</strong>), 95% of salt destined for human c<strong>on</strong>sumpti<strong>on</strong>must be iodized according to government standards for iodine c<strong>on</strong>tent.• Salt iodine c<strong>on</strong>centrati<strong>on</strong>s at the point of producti<strong>on</strong> or importati<strong>on</strong>, and at the wholesaleand retail levels, must be determined by titrati<strong>on</strong>; at the household level, it may be determinedby either titrati<strong>on</strong> or certified kits.bData (nati<strong>on</strong>al or regi<strong>on</strong>al) should have been collected <strong>with</strong>in the last 2 years.Source: adapted from reference (1).Reference1. Assessment of iodine deficiency disorders and m<strong>on</strong>itoring their eliminati<strong>on</strong>. A guide forprogramme managers. 2nd ed. Geneva, World Health Organizati<strong>on</strong>, 2001(WHO/NHD/01.1).286
ANNEX BThe internati<strong>on</strong>al resource laboratory foriodine networkThe Internati<strong>on</strong>al Resource Laboratory for Iodine network (IRLI), launched in2001, is sp<strong>on</strong>sored by the Centers for Disease C<strong>on</strong>trol and Preventi<strong>on</strong> (CDC),the Internati<strong>on</strong>al Council for C<strong>on</strong>trol of Iodine Deficiency Disorders(ICCIDD), the Micr<strong>on</strong>utrient Initiative (MI), the United Nati<strong>on</strong>s Children’sFund (UNICEF) and the World Health Organizati<strong>on</strong> (WHO). Its purpose is tosupport the nati<strong>on</strong>al public health and industry m<strong>on</strong>itoring that c<strong>on</strong>tributes tosustaining progress towards achieving universal salt iodizati<strong>on</strong> and the eliminati<strong>on</strong>of iodine deficiency 1 .The global IRLI network works to strengthen the capacity of participatinglaboratories to accurately measure iodine in urine and salt. Its main activitiesinclude:(i)(ii)training and technology transfer to nati<strong>on</strong>al laboratories;formati<strong>on</strong> of regi<strong>on</strong>al iodine networks;(iii) development of technical standards and external quality assurance/proficiencytesting programmes;(iv) collaborati<strong>on</strong> <strong>with</strong> the salt industry and other sectors when appropriate;(v)informati<strong>on</strong> sharing am<strong>on</strong>g regi<strong>on</strong>al networks and communicati<strong>on</strong>s <strong>with</strong>the IRLI Coordinating Committee and other interested parties;(vi) seeking necessary resources to sustain the operati<strong>on</strong> of regi<strong>on</strong>al networks.As of 2004?? membership of the Internati<strong>on</strong>al Resource Laboratory for Iodinenetwork extended to 12 countries, as follows:AustraliaInstitute of Clinical Pathology and Medical ResearchWestmead HospitalDarcy Road1More informati<strong>on</strong> <strong>on</strong> the IRLI network can be obtained by e-mailing: iodinelab@cdc.gov.287
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSWestmeadNew South Wales 2145http://www.wsahs.nsw.gov.au/icpmrBelgiumCentre Hospitalier Universitaire Saint-Pierre322 Rue Haute1000 Brusselse-mail: Daniella_GNAT@stpierre-bru.beBulgariaNati<strong>on</strong>al Center of Hygiene, Medical Ecology and Nutriti<strong>on</strong>15 Dimiter Nestorov StreetFloor 6, Laboratory 5–6Sofia 1431http://www.nchmen.government.bgCamero<strong>on</strong>Faculty of Medicine and Biomedical SciencesBP 1364Sciences – FMBSYaoundee-mail: WHO.YAO@camnet.cmChinaNati<strong>on</strong>al Reference Laboratory for Iodine Deficiency DisordersDisease C<strong>on</strong>trol DepartmentMinistry of HealthPO Box No 5ChangpingBeijing 102206e-mail: nrl@cnidd.orgGuatemalaFood Safety and Fortificati<strong>on</strong> AreaInstituto de Nutrición de Centro América y Panamá (INCAP)Calzada Roosevelt, Z<strong>on</strong>a 11Apartado Postal 1188Guatemala Cityhttp://www.incap.ops-oms.org288
B. THE INTERNATIONAL RESOURCE LABORATORY FOR IODINE NETWORKIndiaAll India Institute of Medical SciencesCentre for Community MedicineRoom 28New Delhi – 110 029e-mail: cpandav@now-india.net.inInd<strong>on</strong>esiaLaboratorium Biotehnologi Kedokteran/GAKYDip<strong>on</strong>egoro Medical FacultyGedung Serba Guna Lantai 2Jalan Dr Sutomo No. 14KedokteranSemarange-mail: hertanto@indosat.net.idKazakhstanThe Kazakh Nutriti<strong>on</strong> InstituteKlochkov Str. 66Almaty 480008e-mail: nutrit@nursat.kzPeruUnidad de Endocrinologia y MetabolismoInstituto de Investigaci<strong>on</strong>es de la AlturaUniversidad Peruana Cayetano HerediaAv. H<strong>on</strong>orio Delgado 430San Martin de PorresLima 1e-mail: epretell@terra.com.peRussiaInstitute of EndocrinologyDm Ulyanova, 11Moscowe-mail: iod@endocrincentr.ru289
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSSouth AfricaNutriti<strong>on</strong>al Interventi<strong>on</strong> Research UnitMedical Research CouncilPO Box 19070Tygerberg 7505Cape Towne-mail: pieter.jooste@mrc.ac.za290
ANNEX CC<strong>on</strong>versi<strong>on</strong> factors for calculatingEstimated Average Requirements (EARs)from FAO/WHO Recommended NutrientIntakes (RNIs)The recommended method for setting fortificant levels in <strong>food</strong>s is the EstimatedAverage Requirement cut-point method (1). Estimated Average Requirements(EARs) for use in such computati<strong>on</strong>s can be derived from published RecommendedNutrient Intakes (RNIs), by the applicati<strong>on</strong> of the c<strong>on</strong>versi<strong>on</strong> factorslisted in the table below. The EAR is obtained by dividing the RNI (or an equivalentdietary reference value) for a given populati<strong>on</strong> subgroup by the corresp<strong>on</strong>dingc<strong>on</strong>versi<strong>on</strong> factor (Table C.1).The c<strong>on</strong>versi<strong>on</strong> is equivalent to subtracting 2 standard deviati<strong>on</strong>s of theaverage nutrient requirement for a populati<strong>on</strong> subgroup. The c<strong>on</strong>versi<strong>on</strong> factorslisted here are based <strong>on</strong> standard deviati<strong>on</strong>s derived by the United States Foodand Nutriti<strong>on</strong> Board of the Institute of Medicine (FNB/IOM) and which areused by the Board to calculate its Recommended Dietary Allowances (RDAs).291
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE C.1C<strong>on</strong>versi<strong>on</strong> factors for calculating Estimated Average Requirements (EARs) from FAO/WHO Recommended Nutrient Intakes(RNIs)Nutrient Children Males Females1–3 4–6 7–9 10–18 19–65 >65 10–18 19–50 51–65 >65 Pregnant Lactatingyears years years years years years years years years yearsVitamin A 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4Vitamin D a – – – – – – – – – – – –Vitamin E 1.25 1.25 1.25 1.25 1.3 1.3 1.25 1.2 1.2 1.2 1.2 1.2Vitamin C 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.3 1.3 1.2 1.2 1.2Thiamine (vitamin B1) 1.25 1.25 1.25 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2Ribiflavin (vitamin B2) 1.25 1.25 1.25 1.2 1.2 1.2 1.1 1.2 1.2 1.2 1.2 1.2Niacin 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3 1.3Vitamin B6 1.25 1.25 1.25 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2Folate 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25 1.25Vitamin B12 1.3 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2Ir<strong>on</strong> b – – – 1.4 1.3 1.3 1.6 – 1.6 1.6 1.2 1.4Zinc 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2Calcium c 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2Selenium 1.2 1.3 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2 1.2Iodine 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4 1.4Fluoride a – – – – – – – – – – – –aC<strong>on</strong>versi<strong>on</strong> factors are not given for vitamin D and fluoride because there is insufficient informati<strong>on</strong> to support the derivati<strong>on</strong> of an EAR for thesemicr<strong>on</strong>utrients. The recommended intakes are usually expressed as Adequate Intakes (AIs), or represented by the usual intake of healthy people.bC<strong>on</strong>versi<strong>on</strong> factors are not provided for children ≤9 years or for menstruating women aged 19–50 years, and should not be used for women aged14–18 years who are menstruating, due to the high variability and the skewed nature of the distributi<strong>on</strong> of the requirements for ir<strong>on</strong> in these populati<strong>on</strong>groups.cC<strong>on</strong>versi<strong>on</strong> factors to be applied to calcium requirements set by the United Kingdom Department of Health (i.e. Reference Nutrient Intakes), whichare c<strong>on</strong>ceptually similar to the FAO/WHO RNIs. (2)Source: reference (1).292
C. CONVERSION FACTORS FOR CALCULATING ESTIMATED AVERAGE REQUIREMENTSReferences1. Food and Nutriti<strong>on</strong> Board, Institute of Medicine. Dietary reference intakes: applicati<strong>on</strong>sin dietary planning. Washingt<strong>on</strong>, DC, Nati<strong>on</strong>al Academy Press, 20032. Department of Health. Dietary Reference Values of <strong>food</strong> energy and nutrients for theUnited Kingdom. L<strong>on</strong>d<strong>on</strong>, Her Majesty’s Stati<strong>on</strong>ery Office, 1991.293
ANNEX DA procedure for estimatingfeasible fortificati<strong>on</strong> levels for amass fortificati<strong>on</strong> programme1. Introducti<strong>on</strong>Mass fortificati<strong>on</strong> is the term used to describe the additi<strong>on</strong> of micr<strong>on</strong>utrients to<strong>food</strong>s that are widely c<strong>on</strong>sumed, such as staples, c<strong>on</strong>diments and several othercommodities. This can be a very efficient way of supplying micr<strong>on</strong>utrients to alarge proporti<strong>on</strong> of the target populati<strong>on</strong> for a number of reas<strong>on</strong>s. Firstly, massfortificati<strong>on</strong> does not require changes in dietary habits and sec<strong>on</strong>dly, programmescan be based <strong>on</strong> existing <strong>food</strong> distributi<strong>on</strong> networks. In additi<strong>on</strong>,staples and c<strong>on</strong>diments tend to be c<strong>on</strong>sumed throughout the year, and whenfortified <strong>on</strong> an industrial scale, the increase in the cost of the product due to fortificati<strong>on</strong>is usually relatively small. On the downside, because staples and c<strong>on</strong>dimentsare also c<strong>on</strong>sumed in large amounts by n<strong>on</strong>-target groups, when fortified,some individuals could be put at risk of increasing their nutrient intakes to levelsthat are close to, or exceed, the Tolerable Upper Intake Level (UL). This can bea potential problem for nutrients such as vitamin A, vitamin D, vitamin C, niacin(when using nicotinic acid as the fortificant), folic acid, ir<strong>on</strong>, zinc, calcium,iodine and fluoride.In practice, the amount of fortificant micr<strong>on</strong>utrient that can be added to a<strong>food</strong> is often dictated by safety c<strong>on</strong>cerns for those at the top end of c<strong>on</strong>sumpti<strong>on</strong>of the chosen <strong>food</strong> vehicle. In additi<strong>on</strong>, some micr<strong>on</strong>utrients, including β-carotene, vitamin C, riboflavin (vitamin B 2 ), ir<strong>on</strong>, zinc, calcium and iodine, can<strong>on</strong>ly be added in amounts up to a certain threshold, bey<strong>on</strong>d which the sensoryproperties of the <strong>food</strong> vehicle are negatively affected. Fortificati<strong>on</strong> levels can alsobe restricted by the cost of the added micr<strong>on</strong>utrients; high fortificant costs mightmean that programmes are unaffordable or at risk of not being implemented asplanned. Vitamin A (n<strong>on</strong>-oily), vitamin D, vitamin C, niacin and some compoundsof ir<strong>on</strong> and calcium are am<strong>on</strong>g those nutrients whose additi<strong>on</strong> to <strong>food</strong>are most likely to be limited by cost c<strong>on</strong>straints. In sum, such limitati<strong>on</strong>s <strong>on</strong> themagnitude of micr<strong>on</strong>utrient additi<strong>on</strong>s need to be balanced against the desire toachieve a particular nutriti<strong>on</strong>al goal.For this reas<strong>on</strong>, when planning a mass fortificati<strong>on</strong> programme, or morespecifically, when deciding <strong>on</strong> the level of fortificati<strong>on</strong>, it is advisable to first294
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELSdetermine the probable safety, technological and cost c<strong>on</strong>straints <strong>on</strong> the amountof micr<strong>on</strong>utrient that can be added to a given <strong>food</strong> vehicle. Having establisheda limiting level for each of these factors, the “lowest” value of the three thenbecomes what is referred to as the Feasible Fortificati<strong>on</strong> Level (FFL). A methodologyfor determining the FFL is described in secti<strong>on</strong> 2 below, and its applicati<strong>on</strong>illustrated by means of a worked example in secti<strong>on</strong> 3.The Feasible Fortificati<strong>on</strong> Level (FFL) is that which is determined, subject tocost and technological c<strong>on</strong>straints, as the level that will provide the greatestnumber of at-risk individual <strong>with</strong> an adequate micr<strong>on</strong>utrient intake <strong>with</strong>outcausing an unacceptable risk of excess intake in the whole populati<strong>on</strong>.The FFL is a useful c<strong>on</strong>cept in that it can be used to estimate the additi<strong>on</strong>alintake that would result from the c<strong>on</strong>sumpti<strong>on</strong> of a given amount of a fortified<strong>food</strong>, to decide the final formulati<strong>on</strong> of a micr<strong>on</strong>utrient premix, and toestimate the cost of fortificati<strong>on</strong> for each micr<strong>on</strong>utrient added. The FFL is usedas the basis for various producti<strong>on</strong> and regulatory parameters that are comm<strong>on</strong>lyassociated <strong>with</strong> <strong>food</strong> fortificati<strong>on</strong>. Producti<strong>on</strong> parameters are applied at <strong>food</strong>processing factories, and include the Target Fortificati<strong>on</strong> Level (TFL), theMaximum Fortificati<strong>on</strong> Level (MFL), and the Minimum Fortificati<strong>on</strong> Level(mFL). The latter is used in nati<strong>on</strong>al <strong>food</strong> regulati<strong>on</strong> to establish the LegalMinimum Level (LmL). Another important regulatory parameter is theMaximum Tolerable Level (MTL), which is invoked in <strong>food</strong> law for thosenutrients whose intake might approach the UL as a result of fortificati<strong>on</strong> (seesecti<strong>on</strong> 2.4). Figure D1 illustrates the relati<strong>on</strong>ship between the producti<strong>on</strong> andregulatory parameters defined here.The Target Fortificati<strong>on</strong> Level (TFL) is the average micr<strong>on</strong>utrient c<strong>on</strong>centrati<strong>on</strong>of a fortified <strong>food</strong> product measured at the factory. Food factories should aimto produce products that c<strong>on</strong>tain this target level. It is calculated by adding thenatural intrinsic c<strong>on</strong>centrati<strong>on</strong> of each micr<strong>on</strong>utrient in the unfortified <strong>food</strong>vehicle to the FFL.The Minimum Fortificati<strong>on</strong> Level (mFL) is given by reducing the TFL by anamount equivalent to two coefficients of variati<strong>on</strong> in the measured micr<strong>on</strong>utrientc<strong>on</strong>tent of a fortified <strong>food</strong> at the factory. This level represents the lower limitof the micr<strong>on</strong>utrient c<strong>on</strong>tent to be achieved by the fortificati<strong>on</strong> process.295
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSFIGURE D1Relati<strong>on</strong>ship between the various producti<strong>on</strong> and regulatory parametersassociated <strong>with</strong> mass fortificati<strong>on</strong>FFLIntrinsic c<strong>on</strong>tentLosses indistributi<strong>on</strong>andmarketingVariati<strong>on</strong>HmL mFL TFL MFLLmLFFL = Feasible Fortificati<strong>on</strong> Level; mFL = Minimum Fortificati<strong>on</strong> Level (a producti<strong>on</strong>parameter); TFL = Target Fortificati<strong>on</strong> Level (a producti<strong>on</strong> parameter); MFL =Maximum Fortificati<strong>on</strong> Level (a producti<strong>on</strong> parameter); LmL = Legal Minimum Level(a regulatory parameter), MTL = Maximum Tolerable Level (a regulatory parameter).The graph also shows the Household Minimum Level (HmL), which may be lowerthan the LmL, <strong>on</strong> account of losses during storage in the home (i.e. before the <strong>food</strong>is c<strong>on</strong>sumed). This parameter is sometimes used to m<strong>on</strong>itor the utilizati<strong>on</strong>, coverageand c<strong>on</strong>sumpti<strong>on</strong> of fortified <strong>food</strong>s by c<strong>on</strong>sumers.MTLThe Maximum Fortificati<strong>on</strong> Level (MFL) is given by increasing the TFL by anamount equivalent to two coefficients of variati<strong>on</strong> of the measured micr<strong>on</strong>utrientc<strong>on</strong>tent of a fortified <strong>food</strong> at the factory. This level represents the upper limitof the micr<strong>on</strong>utrient c<strong>on</strong>tent to be achieved by the fortificati<strong>on</strong> process.The Legal Minimum Level (LmL) is the minimum micr<strong>on</strong>utrient c<strong>on</strong>tent of a fortified<strong>food</strong> as defined in regulati<strong>on</strong>s and standards; it is the amount that shouldappear <strong>on</strong> the label of a fortified <strong>food</strong>. The LmL is obtained by reducing themFL by an amount equivalent to the average loss of micr<strong>on</strong>utrient during distributi<strong>on</strong>and storage, <strong>with</strong>in the stated shelf-life of the product.296
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELSThe Maximum Tolerable Level (MTL) is the maximum micr<strong>on</strong>utrient c<strong>on</strong>tent thata fortified <strong>food</strong> can present as it is established in <strong>food</strong> law; its purpose is tominimize the risk of excess intake of certain micr<strong>on</strong>utrients. The MTL shouldcoincide <strong>with</strong> the MFL for those micr<strong>on</strong>utrients for which there is a risk of excessintake.2. Selecting fortificati<strong>on</strong> levels <strong>on</strong> the basis of safety,technological and cost c<strong>on</strong>straints2.1 Limits to micr<strong>on</strong>utrient additi<strong>on</strong>s2.1.1 The safety limitMicr<strong>on</strong>utrient intake is a functi<strong>on</strong> of the amount of <strong>food</strong> c<strong>on</strong>sumed and also themicr<strong>on</strong>utrient c<strong>on</strong>tent of the <strong>food</strong>. Since adult males tend to have the highest<strong>food</strong> c<strong>on</strong>sumpti<strong>on</strong> rates of staple <strong>food</strong>s (and thus the highest micr<strong>on</strong>utrientintakes if a staple were to be mass fortified), this group has the greatest risk ofexcessive micr<strong>on</strong>utrient intakes. In order to assess the risk of excessive intakes,it is necessary to determine the 95th percentile of c<strong>on</strong>sumpti<strong>on</strong> of the <strong>food</strong> tobe fortified, as well as the usual nutrient intake from all dietary sources (includingdietary supplements if they supply nutrient forms that are of c<strong>on</strong>cern froma safety point of view) for those individuals most at risk – in this case, adultmales.Based <strong>on</strong> these assumpti<strong>on</strong>s, the safety limit for a micr<strong>on</strong>utrient additi<strong>on</strong> canbe calculated using Equati<strong>on</strong> 1. Note that if more than <strong>on</strong>e <strong>food</strong> is being c<strong>on</strong>sideredfor mass fortificati<strong>on</strong>, the safety limit should be divided am<strong>on</strong>g all ofthem. If the <strong>food</strong> vehicles to be fortified are interchangeable in the diet (e.g.wheat flour and maize flour, cereals and pastas) the usual intake of the interchangeable<strong>food</strong>s can be combined in order to estimate a comm<strong>on</strong> safety limit,and in turn, a comm<strong>on</strong> Feasible Fortificati<strong>on</strong> Level.Equati<strong>on</strong> 12.1.2 The technological limitA <strong>food</strong> can <strong>on</strong>ly be fortified up to a level that does not change its organoleptic(i.e. colour, flavour, odour) and physical properties, measured just after fortifi-1A more accurate calculati<strong>on</strong> may c<strong>on</strong>sider losses during distributi<strong>on</strong> and storage, as well as lossesduring <strong>food</strong> preparati<strong>on</strong>. However, because losses vary hugely according to c<strong>on</strong>diti<strong>on</strong>s and situati<strong>on</strong>s,and because allowance is often made to compensate for these losses (i.e. an overage), it isusually acceptable to use this simplified approach.297
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTScati<strong>on</strong> and over the shelf-life of the <strong>food</strong>. This level should be determined experimentallyboth for the <strong>food</strong> and for products for which the fortified <strong>food</strong> is animportant ingredient. Ideally, a range of micr<strong>on</strong>utrient levels – and, if more than<strong>on</strong>e micr<strong>on</strong>utrient is involved, combinati<strong>on</strong>s of micr<strong>on</strong>utrient levels – should betested by individuals <strong>with</strong> expertise in the sensory analysis of <strong>food</strong>s in order todetermine what amount of each nutrient is technically compatible <strong>with</strong> a given<strong>food</strong> matrix. Each combinati<strong>on</strong> of micr<strong>on</strong>utrient(s) and <strong>food</strong> matrix will haveits own set of technological maxima. Technological limits are not necessarilyfixed; as a result of technological innovati<strong>on</strong> (e.g. the development of new fortificantsthat have fewer colour, odour and reactive problems), it may well bepossible to raise the technological maximum at a future date.2.1.3 The cost limitOf the three, the cost limit is generally the more flexible and adjustable parameter,being dependent <strong>on</strong> value judgements about what is an acceptable priceincrease for fortified <strong>food</strong> products. Most <strong>on</strong>going <strong>food</strong> fortificati<strong>on</strong> programmesoperate <strong>with</strong> price increases in the range 0.25–2.0%.It is recommended that fortificati<strong>on</strong> programme managers discuss <strong>with</strong> industryat an early stage of programme development what an acceptable incrementin producti<strong>on</strong> costs and product price would be, i.e. <strong>on</strong>e that would make anymass fortificati<strong>on</strong> programme both feasible and sustainable. If more than <strong>on</strong>emicr<strong>on</strong>utrient is to be added, then their combined cost should fall <strong>with</strong>in thispredefined permitted increment.When c<strong>on</strong>ducted <strong>on</strong> a relatively large-scale industrial basis, by far the largestshare of the incremental cost of fortificati<strong>on</strong> (90% or more) can be attributed tothe cost of the fortificant itself. This being the case, the cost limit can be calculatedaccording to Equati<strong>on</strong> 2, where the cost of the fortificant micr<strong>on</strong>utrient(s)is used to substitute for the cost of the entire fortificati<strong>on</strong> programme. Thisapproximati<strong>on</strong> does not apply to some rice fortificati<strong>on</strong> processes, which rely <strong>on</strong>the use of rice premixes in low diluti<strong>on</strong> rates (1:100 or 1:200). In this case, thecost of manufacturing of the premix exceeds that of the fortificant compounds.Equati<strong>on</strong> 21A more accurate calculati<strong>on</strong> may c<strong>on</strong>sider losses during distributi<strong>on</strong> and storage, as well as lossesduring <strong>food</strong> preparati<strong>on</strong>. However, because losses vary hugely according to c<strong>on</strong>diti<strong>on</strong>s and situati<strong>on</strong>s,and because allowance is often made to compensate for these losses (i.e. an overage), it isusually acceptable to use this simplified approach.298
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELS2.2 Estimating the Feasible Fortificati<strong>on</strong> Level (FFL)As stated in the introducti<strong>on</strong>, whichever <strong>on</strong>e of the three limits defined and calculatedas above, i.e. the safety, the technological and the cost limit is the lowestbecomes the FFL. Each micr<strong>on</strong>utrient in a given <strong>food</strong> matrix will have its ownFFL.Once the FFL has been defined, it is possible to estimate for each micr<strong>on</strong>utrientthe additi<strong>on</strong>al intake that would be supplied to the target populati<strong>on</strong>, aswell as the probable cost of the fortificati<strong>on</strong> process (based <strong>on</strong> the cost of thefortificants), and the final formulati<strong>on</strong> of the premix (by multiplying the FFLby the diluti<strong>on</strong> factor).2.3 Estimating producti<strong>on</strong> parameters: the Target Fortificati<strong>on</strong> Level (TFL),the Minimum Fortificati<strong>on</strong> Level (mFL) and the Maximum Fortificati<strong>on</strong>Level (MFL)The TFL is given by the sum of the calculated FFL and the naturalintrinsic c<strong>on</strong>tent of the micr<strong>on</strong>utrient in the unfortified <strong>food</strong>. The value of theTFL should be used at the factory level as the target average micr<strong>on</strong>utrientc<strong>on</strong>tent of a fortified <strong>food</strong>, and thus as the reference value for quality c<strong>on</strong>trolspecificati<strong>on</strong>s.The mFL is derived from the TFL according to Equati<strong>on</strong> 3, that is to say,the TFL is reduced by an amount that is proporti<strong>on</strong>al to two times the coefficientof variati<strong>on</strong> (CV) of the measured nutrient c<strong>on</strong>tent of a <strong>food</strong> that has beenfortified by a given process (when that process is performing adequately). Thevariability in the micr<strong>on</strong>utrient c<strong>on</strong>tent of a fortified <strong>food</strong> depends <strong>on</strong> the natureof the <strong>food</strong> vehicle and the amount of micr<strong>on</strong>utrient added. Generally speaking,the inherent variability in the fortificati<strong>on</strong> process is lowest for liquids andgreatest for coarse solids. For liquids, a CV of 10% is typical; for fine solids,such as cereal flours, the additi<strong>on</strong> of niacin, ir<strong>on</strong>, zinc and calcium has a CV of15%, which rises to 25% for most other micr<strong>on</strong>utrients.The variability for coarsesolids, such as sugar and unrefined salt, is higher still, generally speaking around30–50%.Equati<strong>on</strong> 3mFL (mg/kg) = TFL × [1 − (2 × CV in the nutrient c<strong>on</strong>tent of thefortificati<strong>on</strong> process (%/100)]The MFL is calculated in a similar way, the <strong>on</strong>ly difference being that twice theCV of the micr<strong>on</strong>utrient c<strong>on</strong>tent achieved by the fortificati<strong>on</strong> process when performingadequately is added to the TFL (Equati<strong>on</strong> 4):299
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSEquati<strong>on</strong> 4MFL (mg/kg) = TFL × [1 + (2 × CV in the nutrient c<strong>on</strong>tent during thefortificati<strong>on</strong> process (%/100)]2.4 Estimating regulatory parameters: the Legal Minimum Level (LmL)and the Maximum Tolerable Level (MTL)Irrespective of whether mass fortificati<strong>on</strong> is mandatory or voluntary, from apublic health perspective, fortificati<strong>on</strong> levels should be prescribed in nati<strong>on</strong>alstandards and regulati<strong>on</strong>s. Such regulati<strong>on</strong>s may menti<strong>on</strong> the technologicalparameters described in secti<strong>on</strong> 2.3, but it is essential that they refer to thoselevels that should feature <strong>on</strong> <strong>food</strong> labels and which should be used for inspecti<strong>on</strong>and enforcement purposes, i.e. the LmL and the MTL.The LmL is calculated by subtracting from the mFL the expected losses ofmicr<strong>on</strong>utrients during the distributi<strong>on</strong> and storage of fortified products. Equati<strong>on</strong>5 summarizes the calculati<strong>on</strong>:Equati<strong>on</strong> 5LmL (mg/kg) = [mFL (mg/kg) (1 − proporti<strong>on</strong> of lossesduring storage and distributi<strong>on</strong>)]It may be necessary to specify a time frame after fortificati<strong>on</strong>, during which timenutriti<strong>on</strong>al claims must be upheld. In general, most mineral c<strong>on</strong>tents, <strong>with</strong> theexcepti<strong>on</strong> of iodine in raw salt, should remain more or less c<strong>on</strong>stant, but vitaminc<strong>on</strong>tents are more liable to change <strong>with</strong> time, depending <strong>on</strong> the product.However, such losses rarely exceed 50%, even for the most sensitive nutrients(e.g. vitamin A, folic acid) during the shelf-life of the fortified <strong>food</strong>.The MTL is simply the legal expressi<strong>on</strong> of the MFL for those nutrients forwhich there may be a safety c<strong>on</strong>cern, for example, vitamin A, vitamin D, folicacid, niacin (as nicotinic acid) ir<strong>on</strong>, zinc, calcium and iodine. For other nutrientsit may not be necessary to specify this parameter in regulati<strong>on</strong>s, somethingwhich reduces the complexity of the enforcement system required.3. Selecting a fortificati<strong>on</strong> level based <strong>on</strong> the FFL: an examplecalculati<strong>on</strong>A government of a country is aware that most of its populati<strong>on</strong> has a diet richin cereals but poor in <strong>food</strong>s of animal origin. C<strong>on</strong>sequently, the general populati<strong>on</strong>is at risk of deficiencies in vitamin A, riboflavin (vitamin B 2 ), folate,vitamin B 12 , ir<strong>on</strong> and zinc. The government is c<strong>on</strong>sidering introducing a massfortificati<strong>on</strong> programme to counteract the risk of multiple micr<strong>on</strong>utrientdeficiencies and to this end has requested its public health nutriti<strong>on</strong>ists to300
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELSinvestigate the feasibility of supplying 70% of the Estimated Average Requirements(EARs) of these micr<strong>on</strong>utrients via fortified <strong>food</strong>s and to recommendsuitable fortificati<strong>on</strong> levels for achieving this nutriti<strong>on</strong>al goal.3.1 Selecting appropriate <strong>food</strong> vehicles and determining the significanceof <strong>food</strong> fortificati<strong>on</strong> in public health termsData <strong>on</strong> the level of c<strong>on</strong>sumpti<strong>on</strong> am<strong>on</strong>g the target populati<strong>on</strong> of four widelyc<strong>on</strong>sumed staples, sugar, oil, wheat flour and rice, are summarized in Table D.1.On the grounds that they are c<strong>on</strong>sumed by at least 50% of the populati<strong>on</strong>,sugar, oil and wheat flour were singled out as being the most appropriate vehiclesfor mass fortificati<strong>on</strong>. Although rice is also c<strong>on</strong>sumed in large amounts bythe populati<strong>on</strong>, much of the supply is produced at small-scale, local mills, andthus much more difficult to fortify.Although reas<strong>on</strong>able coverage can be achieved by the fortificati<strong>on</strong> of the threenominated <strong>food</strong> vehicles, there was nevertheless some c<strong>on</strong>cern that up to 30%of the target populati<strong>on</strong> might not benefit from the planned fortificati<strong>on</strong> programme.The sector of the populati<strong>on</strong> falling into this category is that whichresides in rural areas and whose accessibility to industrially-processed <strong>food</strong>s islikely to be limited. Since it is technically possible to add vitamin A to all threevehicles, coverage is likely to be the greatest for this vitamin. However, for someof the other nutrients under c<strong>on</strong>siderati<strong>on</strong>, which can <strong>on</strong>ly be easily added to<strong>on</strong>e of the three proposed vehicles (i.e. wheat flour), coverage is likely to be significantlylower. It was c<strong>on</strong>cluded that the potential coverage made vitamin Afortificati<strong>on</strong> of all three products worthwhile, but that it would be necessary toprovide micr<strong>on</strong>utrient supplements in various forms (e.g. tablets, powders, beverages)to ensure an adequate micr<strong>on</strong>utrient intake by that fracti<strong>on</strong> of the populati<strong>on</strong>not covered by mass fortificati<strong>on</strong> (in particular those living in ruralareas). It was recommended that supplements be distributed both commerciallyand through social programmes, and that they should provide the equivalent of70% of the EAR for the micr<strong>on</strong>utrients of c<strong>on</strong>cern. The proposed compositi<strong>on</strong>TABLE D.1C<strong>on</strong>sumpti<strong>on</strong> profile of selected industrially-produced staplesFood C<strong>on</strong>sumers (% of populati<strong>on</strong>) C<strong>on</strong>sumpti<strong>on</strong> a (g/day)P-5th P-50th P-95 thSugar 70 10 20 60Oil 60 5 10 25Wheat flour 50 100 200 600Rice b 10 100 250 700abExpressed as percentiles of c<strong>on</strong>sumpti<strong>on</strong>.Refers to rice produced at larger-scale industrial facilities <strong>on</strong>ly.301
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE D.2Recommended compositi<strong>on</strong> of dietary supplementsto complement fortified <strong>food</strong>sMicr<strong>on</strong>utrientDaily equivalent dose aVitamin A 300 µgvitamin B 2 (riboflavin)0.8 mgFolic acid200 µg bVitamin B 121.4 µg cIr<strong>on</strong>10 mgZinc4 mgabcThese doses are given as equivalent doses so that they canbe used to formulate a daily as well as a disc<strong>on</strong>tinuous dose(e.g. a weekly dose). The aim is to supply at least 70% EARfor adult males, which is used as the reference average forthe family.200 µg folic acid is equivalent to 340 µg Dietary Folate Equivalents(200 × 1.7), which means that a dietary supplementc<strong>on</strong>taining this dose would c<strong>on</strong>tribute 106% of the EstimatedAverage Requirement (EAR) for this particular nutrient.This dosage could provide up to 140% of the EstimatedAverage Requirement (EAR) of vitamin B 12 in view of thehigher bioavailability of the synthetic form relative to naturaldietary sources.of the dietary supplements (expressed as daily equivalent doses) are presentedin Table D.2.3.2 Analysing the safety, technological and cost limits tovitamin A fortificati<strong>on</strong>The calculati<strong>on</strong> of a safety limit for vitamin A fortificati<strong>on</strong> needs to take accountof the fact that this micr<strong>on</strong>utrient is to be added to more than <strong>on</strong>e <strong>food</strong> (in thiscase three). Thus as a first step in the calculati<strong>on</strong>, it is necessary to adjust theUL that will be used for the estimati<strong>on</strong> of the safety limit for each <strong>food</strong> as follows:UL per <strong>food</strong> = [UL − (diet and supplement intake)]/3The intake of vitamin A (in the retinol form) from dietary sources by the targetpopulati<strong>on</strong> was estimated to be around 600µg per day. This value represents thehigh end of c<strong>on</strong>sumpti<strong>on</strong> (i.e. the 95th percentile of intakes). Given that the ULfor vitamin A is 3 000µg and assuming a further daily intake of vitamin fromsupplements of 300µg (see Table D.2), then:that is:UL per <strong>food</strong> = [3 000 − (600 + 300)]/3,302
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELSTABLE D.3Safety limits for vitamin AFood 95th percentile of Safety limitc<strong>on</strong>sumpti<strong>on</strong> (g/day)(mg/kg)Sugar 60 12Oil 25 28Wheat flour 300 1.2UL per <strong>food</strong> = 700µg.Then, using Equati<strong>on</strong> 1, it is possible to calculate a safety limit for each <strong>food</strong>.The results are given in Table D.3.The questi<strong>on</strong> then arises whether or not it is technologically feasible to addthese levels of vitamin A to the chosen <strong>food</strong> vehicles. According to the country’s<strong>food</strong> technologists it is, and thus it was c<strong>on</strong>cluded that vitamin A fortificati<strong>on</strong> isunlikely to be limited by technological c<strong>on</strong>siderati<strong>on</strong>s in this scenario.As <strong>food</strong> technologists warned that price increases in <strong>food</strong> products due tofortificati<strong>on</strong> in excess of 2% for sugar and oil, and 0.3% for wheat flour, mightmeet <strong>with</strong> oppositi<strong>on</strong> from the <strong>food</strong> industry, it was c<strong>on</strong>sidered instructive atthis point to estimate the increase in price that would result from fortificati<strong>on</strong>of the three products at the safety limits of vitamin A additi<strong>on</strong>. Table D.4 summarizesthe results of such calculati<strong>on</strong>s.On the basis of these computati<strong>on</strong>s, it is evident that the additi<strong>on</strong> of vitaminA to sugar at a level of 12 mg/kg is barely cost compatible. On the other hand,of the three <strong>food</strong> vehicles, sugar has the best penetrati<strong>on</strong> (see Table D.1). Onbalance, it was decided to proceed <strong>with</strong> the fortificati<strong>on</strong> of sugar, despite the factthat the relative high cost might make the implementati<strong>on</strong> of this interventi<strong>on</strong>much more difficult.3.2.1 Assessing the nutriti<strong>on</strong>al implicati<strong>on</strong>s of the fortificati<strong>on</strong> <strong>with</strong> vitaminA at the Feasible Fortificati<strong>on</strong> LevelsThe probable additi<strong>on</strong>al intakes of vitamin A due to fortificati<strong>on</strong> at the safetylimits calculated above, at the 5th, 50th and 95th percentiles of c<strong>on</strong>sumpti<strong>on</strong> ofeach <strong>food</strong>, are shown in Table D.5. In each case, the additi<strong>on</strong>al intake isexpressed as a percentage of the EAR, which for adult males is 429µg per day.According to the figures given in Table D.5, use of a three-<strong>food</strong> strategywould provide an additi<strong>on</strong>al intake somewhere between 28% 1 and 499% of the1This value corresp<strong>on</strong>ds to the additi<strong>on</strong>al intake of vitamin A at the 5th percentile c<strong>on</strong>sumpti<strong>on</strong>of fortified sugar, which is the <strong>food</strong> <strong>with</strong> the widest c<strong>on</strong>sumpti<strong>on</strong> (70% of the populati<strong>on</strong>).303
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE D.4Cost analysis of fortificati<strong>on</strong> <strong>with</strong> vitamin A at the estimated safety limits forsugar, oil and wheat flourFood Level of vitamin A Cost analysisadditi<strong>on</strong> (mg/kg)Cost of fortificati<strong>on</strong> Product price Price increment(US$ per MT a ) (US$/kg) (%)Sugar 12 11.00 0.50 2.0Oil 28 6.00 0.70 0.9Wheat flour 1.2 0.67 0.45 0.15aMT stands for metric t<strong>on</strong> or 1 000 kg.TABLE D.5Additi<strong>on</strong>al intake of vitamin A at various levels of c<strong>on</strong>sumpti<strong>on</strong> of fortified<strong>food</strong>sFood Level of vitamin A additi<strong>on</strong> (mg/kg) Additi<strong>on</strong>al intake(as a % of the EAR a )P-5th P-50th P-95thSugar 12 28 56 168Oil 28 33 65 163Wheat flour 1.2 28 56 168TOTAL 89 177 499EAR, Estimated Average Requirement.aBased <strong>on</strong> the EAR of vitamin A for adult males (429 µg/day). This value is used to representthe “average” intake for the family.EAR for adult males (i.e. the extreme values of this combined strategy). Thisfinding provides justificati<strong>on</strong> for the decisi<strong>on</strong> to proceed <strong>with</strong> the vitamin A fortificati<strong>on</strong>of sugar (despite the cost) as <strong>with</strong>out it, the programme is unlikely toattain its nutriti<strong>on</strong>al goal of supplying 70% of the EAR to most individuals inthe populati<strong>on</strong>.The above analysis also dem<strong>on</strong>strates the benefits of fortifying three vehicles<strong>with</strong> lower amounts of vitamin A rather than just <strong>on</strong>e <strong>with</strong> a relatively highamount. Adopting the latter approach would not <strong>on</strong>ly result in an unacceptablyhigh cost increment, but also increases the risk of those individuals at the highend of c<strong>on</strong>sumpti<strong>on</strong> of the single vehicle reaching the UL <strong>with</strong>out significantlyimproving the intake of those individuals at the low end of c<strong>on</strong>sumpti<strong>on</strong>. Furthermore,the coverage of the interventi<strong>on</strong> would be limited to those c<strong>on</strong>sumingthe single chosen <strong>food</strong> vehicle.Taking into account all of the above c<strong>on</strong>siderati<strong>on</strong>s, it was decided to selectthe safety limits of vitamin A fortificati<strong>on</strong> as the FFLs, i.e. for sugar, 12 mg/kg,for oil, 28 mg/kg and for wheat flour: 1.2 mg/kg.304
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELSTABLE D.6Producti<strong>on</strong> parameters for vitamin A fortificati<strong>on</strong>Food FFL Intrinsic vitamin A TFL a CV b mFL c MFL d(mg/kg) c<strong>on</strong>tent (mg/kg) (mg/kg) (%) (mg/kg) (mg/kg)Sugar 12 0.0 12 33 4 20Oil 28 0.0 28 10 22 34Wheat flour 1.2 0.0 1.2 25 0.6 1.8FFL, Feasible Fortificati<strong>on</strong> Level; TFL, Target Fortificati<strong>on</strong> Level; CV, coefficient of variati<strong>on</strong>; mFL,Minimum Fortificati<strong>on</strong> Level; MFL, Maximum Fortificati<strong>on</strong> Level.aThe Target Fortificati<strong>on</strong> Level is given by adding the intrinsic vitamin A c<strong>on</strong>tent of the <strong>food</strong>vehicles to the FFL.bThe coefficient of variati<strong>on</strong> (CV) is a measure of the reproducibility of the fortificati<strong>on</strong> process.cCalculated using Equati<strong>on</strong> 3.dCalculated using Equati<strong>on</strong> 4.TABLE D.7Regulatory parameters for vitamin A fortificati<strong>on</strong>Food FFL Losses during distributi<strong>on</strong> LmL a MTL b(mg/kg) and storage (%) (mg/kg) (mg/kg)Sugar 12 30 3 20Oil 28 30 15 34Wheat flour 1.2 25 0.5 1.8FFL, Feasible Fortificati<strong>on</strong> Level; LmL, Legal minimum Level; MTL, Maximum Tolerable Level.aCalculated using Equati<strong>on</strong> 5.bIn this case, this is the same as the Maximum Fortificati<strong>on</strong> Level (MFL) given in Table D.6.3.2.2 Establishing the producti<strong>on</strong> parametersHaving selected the FFLs, and using the definiti<strong>on</strong>s and equati<strong>on</strong>s given insecti<strong>on</strong> 2.3, the next task is to establish the producti<strong>on</strong> parameters for vitaminA additi<strong>on</strong>s at the factory level. These parameters are given in Table D.6.3.2.3 Establishing the regulatory parametersRegulatory parameters, the LmL and the MTL, for vitamin A fortificati<strong>on</strong> aresummarized in Table D.7. These will form the basis of label claims and governmentenforcement activities. In the case of vitamin A fortificati<strong>on</strong>, it is necessaryto set a MTL because of the need to make sure that individuals <strong>with</strong>inthe populati<strong>on</strong> (i.e. those at the high end of c<strong>on</strong>sumpti<strong>on</strong>) would not be at riskof excessive intakes of vitamin A.3.3 Analysing the safety, technological and cost limits to wheatflour fortificati<strong>on</strong>Having assessed the feasibility of vitamin A additi<strong>on</strong>s, the same procedure canbe repeated to address the questi<strong>on</strong> of the incorporati<strong>on</strong> of folic acid, vitamin305
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSB 12 ,riboflavin (vitamin B 2 ), ir<strong>on</strong> and zinc to wheat flour. Table D.8 provides asummary of the main features of this analysis, which reveals that folic acid additi<strong>on</strong>is limited by safety c<strong>on</strong>cerns, vitamin B 12 additi<strong>on</strong> by cost, and vitamin B 2 ,ir<strong>on</strong> and zinc additi<strong>on</strong>s by the risk of organoleptic changes in the sensorial andphysical properties of the wheat flour.3.3.1 Assessing the nutriti<strong>on</strong>al implicati<strong>on</strong>s of the fortificati<strong>on</strong> of wheatflour, and adjusting the Feasible Fortificati<strong>on</strong> LevelsThe nutriti<strong>on</strong>al implicati<strong>on</strong>s of fortifying wheat flour at the FFLs calculated inTable D.8 (i.e. as determined by safety, technological and cost c<strong>on</strong>straints) aresummarized in Table D.9. This is expressed in terms of the additi<strong>on</strong>al intakesthat will result from the c<strong>on</strong>sumpti<strong>on</strong> of fortified wheat flour at three levels ofc<strong>on</strong>sumpti<strong>on</strong>, the 5th percentile (i.e.100 g per day), the 50th percentile (i.e. 200g per day) and the 95 percentile (i.e. 600 g per day). Intakes are given as absoluteamounts and as a percentage of the EAR for adult males. Please note that thisc<strong>on</strong>sumpti<strong>on</strong> pattern is high, and although it is typical of the Middle East andCentral Asian countries, it may not be the case for other countries of the world.Each regi<strong>on</strong> or country should make their own calculati<strong>on</strong>s based <strong>on</strong> their ownc<strong>on</strong>diti<strong>on</strong>s in order to select the most appropriate fortificati<strong>on</strong> levels.The calculati<strong>on</strong>s show that additi<strong>on</strong> of folic acid to wheat flour would achievethe goal of supplying 70% of the EAR to nearly all c<strong>on</strong>sumers of wheat flour(that is to say, to 50% of the populati<strong>on</strong>). The case of vitamin B 12 is alsofavourable, in fact, particularly so. Its level can be reduced to 0.010 mg/kg (from0.040 mg/kg), which will help to reduce overall cost of the programme while stillsatisfying the nutriti<strong>on</strong>al target (i.e. an additi<strong>on</strong>al intake of 100% of the biologicalrequirements (EAR) of this nutrient for almost all individuals who c<strong>on</strong>sumewheat flour).In c<strong>on</strong>trast, the additi<strong>on</strong> of vitamin B 2 at a level of 4.5 mg per kg is not sufficientto meet nutriti<strong>on</strong>al goals, and therefore other sources of this nutrient (e.g.dietary supplements) would have to be supplied to the target populati<strong>on</strong>. Thesame is true of ir<strong>on</strong>, and, in the case of reproductive-age women the deficit islikely to be even worse, since their ir<strong>on</strong> requirements are greater than those usedin the present calculati<strong>on</strong>.Although fortificati<strong>on</strong> <strong>with</strong> zinc at a level of 40 mg/kg would be expected toattain the EAR goal, in the interests of avoiding possible problems <strong>with</strong> ir<strong>on</strong>absorpti<strong>on</strong> (zinc additi<strong>on</strong>s at these levels could inhibit the absorpti<strong>on</strong> of ir<strong>on</strong>),it was c<strong>on</strong>sidered prudent to reduce the level to 20 mg/kg. This would maintaina suitable balance <strong>with</strong> the additi<strong>on</strong>al ir<strong>on</strong> intake. Any future interventi<strong>on</strong>sshould pair zinc and ir<strong>on</strong> additi<strong>on</strong>s in a way that complements the impact ofwheat flour fortificati<strong>on</strong>.306
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELSTABLE D.8Safety, technological and cost limits for wheat flour fortificati<strong>on</strong> aNutrient Fortificant Cost of Proporti<strong>on</strong> UL Intake from Limits (mg/kg) FFL gfortificant of nutrient (mg/day) b the diet and(mg/kg)(US$/kg) in fortificant supplements Safety d Technological e Cost f(mg/day) cFolate Folic acid 90.00 0.90 1 0.2 1.3 NA 13.5 1.3Vitamin B12 Vitamin B12, 0.1% 38.00 0.001 NA NA NA NA 0.040 0.040water solubleVitamin B2 Riboflavin 38.00 1.00 NA NA NA 4.5 36 4.5Ir<strong>on</strong> Ferrous sulphate, 2.52 0.32 45 10 58 30 171 30driedZinc Zinc oxide 3.35 0.80 45 4 68 40 322 40UL, Tolerable Upper Intake Level; FFL, Feasible Fortificati<strong>on</strong> Level; NA, not applicable.aAssumes that the per capita c<strong>on</strong>sumpti<strong>on</strong> of wheat flour is 100–600 g/day, and that the price of wheat flour is US$ 0.45/kg. This high level of c<strong>on</strong>sumpti<strong>on</strong>is typical of countries in the Middle East and Central Asia. Other countries should calculate their safety values according to their ownc<strong>on</strong>sumpti<strong>on</strong> figures.bValues are for adult males; this group is c<strong>on</strong>sidered to be at greatest risk of reaching the UL through the c<strong>on</strong>sumpti<strong>on</strong> of fortified wheat flour.cIntakes are specified <strong>on</strong>ly for those micr<strong>on</strong>utrients for which there may be a safety c<strong>on</strong>cern (the main source of which, in this case, will be dietarysupplements).dCalculated using Equati<strong>on</strong> 1.eTechnological compatibility is determined experimentally to c<strong>on</strong>firm the absence of undesirable changes in the <strong>food</strong> vehicle due to the additi<strong>on</strong> offortificants.fCalculated using Equati<strong>on</strong> 2. It was predetermined that each nutrient should not increase the price of wheat flour by more than 0.3%.gThe Feasible Fortificati<strong>on</strong> Level (FFL) is the lowest of the three limits.307
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSTABLE D.9Nutriti<strong>on</strong>al implicati<strong>on</strong>s of wheat flour fortificati<strong>on</strong> aNutrient Fortificant FFL EAR b Absolute additi<strong>on</strong>al intakes Additi<strong>on</strong>al intakes (as a % of(mg/kg) (mg/day)(mg/day) the EAR)P-5th P-50th P-95th P-5th P-50th P-95thFolate c Folic acid 1.3 0.32 0.130 0.260 0.780 69 138 414Vitamin B12 d Vitamin B12, 0.1% 0.040 0.002 0.0040 0.0080 0.0240 400 800 2 400water solubleVitamin B12, 0.1% 0.010 e 0.002 0.001 0.002 0.006 100 200 600water solubleVitamin B2 Riboflavin 4.5 1.1 0.45 0.9 2.7 41 82 245Ir<strong>on</strong> f Ferrous sulphate, 30 10 3 6 18 28 56 167driedZinc Zinc oxide 40 5.8 4 8 24 69 138 414Zinc oxide 20 g 5.8 2 4 8 34 69 207FFL, Feasible Fortificati<strong>on</strong> Level; EAR, Estimated Average Requirement.aAssumes that the per capita c<strong>on</strong>sumpti<strong>on</strong> of wheat flour is 100 g/day at the 5th percentile of c<strong>on</strong>sumpti<strong>on</strong>, 200 g/day at the 50th percentile and600 g/day at the 95th percentile.bBased <strong>on</strong> the values for the EAR for adult males. These values are used to represent the “average” intake for the family.cThe calculati<strong>on</strong> of the additi<strong>on</strong>al intake as a percentage of the EAR takes account of the higher bioavailability of folic acid as compared <strong>with</strong> dietaryfolate (1 µg folic acid = 1.7 Dietary Folate Equivalents (DFEs) or 1.7 µg <strong>food</strong> folate).dThe calculati<strong>on</strong> of the additi<strong>on</strong>al intake as a percentage of the EAR takes account of the higher bioavailability of the synthetic form of vitamin B12 ascompared <strong>with</strong> dietary sources (% EAR multiplied by 2).eThe FFL has been adjusted downwards because the original value provided much more than was necessary to attain the nutriti<strong>on</strong>al goal of an additi<strong>on</strong>alintake of 70% of the EAR.fIf the average c<strong>on</strong>sumpti<strong>on</strong> of wheat flour is less than 150 g/day, ferrous fumarate may be used in place of ferrous sulfate as the fortificant in orderto achieve the nutriti<strong>on</strong>al goal of an additi<strong>on</strong>al intake intake of around 50% EAR. However, it is important to note that this change increases ir<strong>on</strong>fortificati<strong>on</strong> costs four-fold.gThe FFL has been adjusted downwards because it was important to keep the nutriti<strong>on</strong>al balance of the diet.308
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELSTABLE D.10Producti<strong>on</strong> and regulatory parameters for wheat flour fortificati<strong>on</strong>Nutrient Fortificant Accepted FFL a Intrinsic CV b Producti<strong>on</strong> parameters Regulatory(mg/kg) c<strong>on</strong>tent (%) parameters(mg/kg) (mg/kg) (mg/kg) LmL f MTL g(mg/kg) MFL c TFL d mFL e(mg/kg) (mg/kg)Folate Folic acid 1.3 0.2 25 0.8 1.5 2.3 0.6 2.3Vitamin B12 Vitamin B12, 0.1% 0.010 0.000 25 0.005 0.010 0.015 0.005 NAwater solubleVitamin B2 Riboflavin 4.5 0.5 25 2.5 5.0 7.5 2.3 NAIr<strong>on</strong> Ferrous sulphate, 30 10 15 28 40 52 28 52driedZinc Zinc oxide 20 10 15 21 30 39 21 39Vitamin A 250-SD 1.2 0 25 0.6 1.2 1.8 0.5 1.8FFL, Feasible Fortificati<strong>on</strong> Level; CV, coefficient of variati<strong>on</strong>; mFL, Minimum Fortificati<strong>on</strong> Level; TFL, Target Fortificati<strong>on</strong> Level; MFL, Maximum Fortificati<strong>on</strong>Level; LmL, Legal minimum Level; MTL, Maximum Tolerable Level; NA, not applicable.aThe level of fortificati<strong>on</strong> that was finally selected, having adjusted the original FFLs for some micr<strong>on</strong>utrients. The compositi<strong>on</strong> of a fortificati<strong>on</strong> premixfor use <strong>with</strong> wheat flours is obtained by multiplying the FFL by the diluti<strong>on</strong> factor.bThe coefficient of variati<strong>on</strong> (CV) is a measure of the reproducibility of the fortificati<strong>on</strong> process.cCalculated using Equati<strong>on</strong> 3.dThe Target Fortificati<strong>on</strong> Level is given by summing the intrinsic micr<strong>on</strong>utrient c<strong>on</strong>tent of the unfortified wheat flour and the FFL. Factories should aimto produce <strong>food</strong>s that, <strong>on</strong> average, c<strong>on</strong>tain this amount of micr<strong>on</strong>utrient.eCalculated using Equati<strong>on</strong> 4.fCalculated using Equati<strong>on</strong> 5.gRelevant <strong>on</strong>ly for those micr<strong>on</strong>utrients <strong>with</strong> safety c<strong>on</strong>cerns; equivalent, in this case, to the MFL.309
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS3.3.2 Establishing producti<strong>on</strong> and regulatory parametersBased <strong>on</strong> the slightly revised FFLs, producti<strong>on</strong> and regulatory parameters forthe fortificati<strong>on</strong> of wheat flour <strong>with</strong> folate, vitamins B 2 and B 12 , ir<strong>on</strong> and zinc arecalculated in the same way as for vitamin A (see secti<strong>on</strong> 3.2.2 and 3.2.3). Theseare given in Table D.10. For completeness, Table D.10 also includes the corresp<strong>on</strong>dingparameters for vitamin A, calculated earlier (Tables D.6 and D.7).3.4 C<strong>on</strong>cluding comments and recommendati<strong>on</strong>sThe above analysis establishes that fortificati<strong>on</strong> of wheat flour at the levels proposed(the “accepted” FFLs) would provide appropriate amounts of essentialmicr<strong>on</strong>utrients to the majority of c<strong>on</strong>sumers. Moreover, the cost of the additi<strong>on</strong>TABLE D.11Final formulati<strong>on</strong> for the fortificati<strong>on</strong> of refined wheat flour and estimatedassociated costs for a hypothetical country aNutrient Fortificant Accepted Regulatory Estimated costs ofFFL parametersfortificati<strong>on</strong>(mg/kg)LmL b MTL c (US$ (% of totalper MT d ) cost)Folate Folic acid 1.3 0.6 2.3 0.13 5.6Vitamin B 12 Vitamin B 12 , 0.1% 0.010 0.005 NA 0.38 16.2water solubleVitamin B 2 Riboflavin 4.5 2.3 NA 0.17 7.3Ir<strong>on</strong> Ferrous sulphate, 30 28 52 0.24 10.1driedZinc Zinc oxide 20 21 39 0.08 3.6Vitamin A 250-SD 1.2 0.5 1.8 0.67 28.7Vitamin B 1 Thiamine m<strong>on</strong><strong>on</strong>itrate 6 2.8 NA 0.18 7.6Vitamin B 6 Pyridoxin 5 2.4 NA 0.17 7.3Niacin Niacinamide 50 40 NA 0.45 13.6Total 2.34 100.0Price increment due to fortificati<strong>on</strong> (%) 0.5FFL, Feasible Fortificati<strong>on</strong> Level; LmL, Legal minimum Level; MTL, Maximum Tolerable Level;NA, not applicable.aAssumes an average per capita c<strong>on</strong>sumpti<strong>on</strong> of wheat flour of 200 g/day (the 95th percentileof c<strong>on</strong>sumpti<strong>on</strong> is 600 g/day), and that the price of wheat flour is US$ 0.45 per kg. This highlevel of c<strong>on</strong>sumpti<strong>on</strong> is typical of countries in the Middle East and Central Asia. Other countriesshould calculate their fortificati<strong>on</strong> formulas according to their own c<strong>on</strong>sumpti<strong>on</strong> figures.bThe Legal Minimum Level (LmL) is the level of fortificant which should appear <strong>on</strong> the labeland is the level to be enforced. It includes the intrinsic nutrient c<strong>on</strong>tent of the unfortified wheatflour.cThe Maximum Tolerable Level is specified for those micr<strong>on</strong>utrients for which there is safetyc<strong>on</strong>cern; its purpose in <strong>food</strong> law is to assure that almost all wheat flour c<strong>on</strong>sumers do notreach the Upper Tolerable Intake Level for the nutrients for which this parameter is specified.dMT stands for metric t<strong>on</strong> or 1 000 kg.310
D. A PROCEDURE FOR ESTIMATING FEASIBLE FORTIFICATION LEVELSTABLE D.12Estimating the overall cost of the proposed fortificati<strong>on</strong> programme and the annual investment requiredFood C<strong>on</strong>sumer Cost of Annual Per capita Per capita C<strong>on</strong>sumpti<strong>on</strong> Total cost d Annual Annualvehicle base fortificati<strong>on</strong> demand c<strong>on</strong>sumpti<strong>on</strong> a c<strong>on</strong>sumpti<strong>on</strong> b per (Milli<strong>on</strong> of investment investment(% of the (US$ per MT g ) (MT g ) (kg/year) (g/day) c<strong>on</strong>sumer c US$ per year) per pers<strong>on</strong> e perpopulati<strong>on</strong>) (g/day) (US$) c<strong>on</strong>sumer f(US$)Sugar 70 11.00 100 000 10 27 39 1.10 0.110 0.157Oil 60 6.00 30 000 3 8 13 0.18 0.018 0.030Wheat flour 50 2.34 500 000 50 137 274 1.17 0.117 0.234Total 2.45 0.245 0.421aThe annual per capita c<strong>on</strong>sumpti<strong>on</strong> (in kg) is calculated by dividing the annual demand by the total populati<strong>on</strong>, which for the purposes of this exampleis assumed to be 10 milli<strong>on</strong> pers<strong>on</strong>s (i.e. annual demand (in MT) × 1 000/10 000 000).bThe daily per capita c<strong>on</strong>sumpti<strong>on</strong> (in g) is calculated by dividing the annual per capita c<strong>on</strong>sumpti<strong>on</strong> by the number of days in the year (i.e. annualper capita c<strong>on</strong>sumpti<strong>on</strong> (in kg) × 1 000/365).cThe daily c<strong>on</strong>sumpti<strong>on</strong> per c<strong>on</strong>sumer is calculated by dividing the daily per capita c<strong>on</strong>sumpti<strong>on</strong> (in g) by the proporti<strong>on</strong> of populati<strong>on</strong> that c<strong>on</strong>sumesthe <strong>food</strong>. Ideally the daily c<strong>on</strong>sumpti<strong>on</strong> per c<strong>on</strong>sumer calculated in this way should equate to between the 50th and 95th percentile of daily c<strong>on</strong>sumpti<strong>on</strong>as determined by dietary surveys.dThe total annual cost of fortificati<strong>on</strong> is calculated as the product of the fortificati<strong>on</strong> cost per MT (in US$) and the annual total demand (in MT).eThe annual investment per pers<strong>on</strong> (in US$) is calculated as the annual total cost (in US$) divided by the total populati<strong>on</strong> (in this example, 10 milli<strong>on</strong>pers<strong>on</strong>s).fThe annual investment per c<strong>on</strong>sumer (in US$) is calculated as the annual investment per pers<strong>on</strong> (in US$) divided by the proporti<strong>on</strong> of the populati<strong>on</strong>that c<strong>on</strong>sumes the <strong>food</strong>.gMT stands for metric t<strong>on</strong> or 1 000 kg.311
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSof vitamin A, vitamin B 2 (riboflavin), folate (folic acid), vitamin B 12 , ir<strong>on</strong> andzinc, was <strong>with</strong>in acceptable limts.Given that the process of milling eliminates many of the B vitamins that arenecessary for the metabolic transformati<strong>on</strong> of starch and protein, and that thecosts associated <strong>with</strong> the additi<strong>on</strong> of these vitamins are relatively small, it wasdecided to include some of the other the B vitamins in the nutrient premix.Table D.11 thus shows the final formulati<strong>on</strong> of the fortified wheat flour, as wellas an estimate of the associated costs.Estimates of the overall cost of the fortificati<strong>on</strong> programme to the country, aswell as the annual investment required per pers<strong>on</strong> and per c<strong>on</strong>sumer, are givenin Table D.12. These figures indicate that the health benefits that can beexpected from the proposal to fortify selected <strong>food</strong>s make the investment anexcellent opti<strong>on</strong> for the country.312
ANNEX EA quality c<strong>on</strong>trol m<strong>on</strong>itoring system forfortified vegetable oils: an example fromMorocco1. BackgroundIn 2002, the Moroccan Ministry of Health launched a programme to fortify vegetableoils <strong>with</strong> vitamins A and D. Prior to its implementati<strong>on</strong>, a Nati<strong>on</strong>al FoodFortificati<strong>on</strong> Committee (NFFC), hosted by the Ministry of Health, was establishedto serve as a forum for the supervisi<strong>on</strong>, follow up and evaluati<strong>on</strong> of theoil fortificati<strong>on</strong> programme in Morocco.This Committee comprised <strong>food</strong> industryrepresentatives, university researchers, staff members of government technicalstandards and inspecti<strong>on</strong> units, and representatives from each of thesp<strong>on</strong>soring agencies.The Committee’s first task was to c<strong>on</strong>duct a feasibility study of soybean oilfortificati<strong>on</strong>. One of the objectives of this study was to determine an appropriatelevel of fortificati<strong>on</strong>, bearing in mind the overages that would be required tocompensate for losses of vitamins A and D 3 during storage and culinary treatment(i.e. cooking and frying). Fortificant levels for vitamins A and D 3 weresubsequently set at 30 IU/g and 3.0 IU/g, respectively, <strong>with</strong> tolerances at theproduct distributi<strong>on</strong> stage in the range of 70–150% of these levels. It was alsoestablished that fortified vegetable oils would need to be commercialized inopaque c<strong>on</strong>tainers.2. Design of the QC/QA systemHaving completed its feasibility study, the Committee reviewed and subsequentlyapproved the proposed quality c<strong>on</strong>trol and quality assurance (QC/QA)procedures for the oil fortificati<strong>on</strong> programme. These procedures, which werebased <strong>on</strong> good manufacturing practice (GMP), were set out in the form of atechnical manual. The technical manual provides comprehensive guidance <strong>on</strong> afull range of m<strong>on</strong>itoring, inspecti<strong>on</strong> and auditing activities but places particularemphasis <strong>on</strong> quality c<strong>on</strong>trol, recognizing this as being a key comp<strong>on</strong>ent of thefortificati<strong>on</strong> programme. In a measure designed to encourage complianceam<strong>on</strong>g producers, fortified oils that had been produced according to the prescribedinternal quality c<strong>on</strong>trol procedures were identified as having been d<strong>on</strong>eso by means of a Ministry of Health logo.313
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS2.1 Hazard analysis and critical c<strong>on</strong>trol pointThe hazard analysis and critical c<strong>on</strong>trol point (HACCP) approach was used asthe basis of the system that was developed for m<strong>on</strong>itoring the quality of fortifiedoils produced in Morocco. The usefulness of this approach for ensuring thesafety of processed <strong>food</strong>s is acknowledged by both the Codex AlimentariusCommissi<strong>on</strong> and the World Health Organizati<strong>on</strong> (WHO). It can also be appliedto the management of the quality of <strong>food</strong> products as this relates to the manufacturingprocess; this makes the HACCP approach complementary to otherquality c<strong>on</strong>trol systems such as the ISO 9001:2000 1 .HACCP analysis is a tool that is used to identify specific hazards (i.e. biological,chemical or physical hazards), as well as preventive measures for eliminatingor c<strong>on</strong>trolling those hazards. In the case of fortified vegetable oils,microbiological hazards are unlikely to be a major c<strong>on</strong>cern, largely because ofthe absence of water in such products. The potential hazards are more likely tobe chemical in nature, for example, c<strong>on</strong>taminati<strong>on</strong> by polyaromatic hydrocarb<strong>on</strong>sor by migrati<strong>on</strong> products from the packaging materials. Quality hazardsmay arise due to problems <strong>with</strong> the refined vegetable oil used as the vehicle forfortificati<strong>on</strong> (e.g. high rates of peroxidati<strong>on</strong>, defects in the flavour characteristics)or <strong>with</strong> the fortificant compounds that are added (e.g. lumping, colour,odour).The seven principles of HACCP, as adopted by Codex (1), establish a frameworkfor developing a HACCP-based system that is specific to a given combinati<strong>on</strong>of <strong>food</strong> product and producti<strong>on</strong> line. Such a system identifies hazards ata series of critical c<strong>on</strong>trol points (CCP), and then for each CCP, identifies criticallimits and appropriate m<strong>on</strong>itoring and c<strong>on</strong>trol measures. The system ismanaged through daily review and analysis of the records for each CCP.It is generally recommended that a HACCP system is periodically evaluatedby an external auditor. In additi<strong>on</strong>, the system should be revised whenever amodificati<strong>on</strong> is made to the producti<strong>on</strong> process, for example, in the wake of customercomplaints or customer surveys that report a product defect.2.2 Critical c<strong>on</strong>trol points in the producti<strong>on</strong> of fortified vegetable oilsApplicati<strong>on</strong> of the HACCP methodology to the producti<strong>on</strong> of fortified oils inMorocco identified the following CCPs; in each case, the appropriate preventivemeasure or acti<strong>on</strong> is described:1ISO 9001:2000 is a norm of the Internati<strong>on</strong>al Standards Organizati<strong>on</strong> for the certificati<strong>on</strong> ofquality management systems in the <strong>food</strong> industry. It signifies adherence to effective qualitysystems to ensure compliance <strong>with</strong> statutory and regulatory requirements applicable to products,and the existence of management reviews, quality objectives and process management focused<strong>on</strong> c<strong>on</strong>tinuous improvement.314
E. A QUALITY CONTROL MONITORING SYSTEM FOR FORTIFIED VEGETABLE OILS1. Receiving of the refined vegetable oils (the <strong>food</strong> vehicle)Acti<strong>on</strong>. Each lot should be tested using approved methods to c<strong>on</strong>firm compliance<strong>with</strong> Moroccan specificati<strong>on</strong>s.2. Quality of the fortificant premixActi<strong>on</strong>. A quality assurance certificate should be obtained from the providerof the premix, and periodic analyses should be c<strong>on</strong>ducted to verify thevitamin c<strong>on</strong>tent as well as the organoleptic properties of the premix (e.g.colour, texture, odour).3. Storage of the fortificant premixActi<strong>on</strong>. The premix should be re-assayed periodically for vitamin c<strong>on</strong>tent toensure that it c<strong>on</strong>tinues to meet the required c<strong>on</strong>centrati<strong>on</strong>s until the endof its shelf-life.4. Additi<strong>on</strong> of the fortificant premixActi<strong>on</strong>. The premix use inventory should be assessed, that is to say, theamount of premix used should be compared <strong>with</strong> the amount of fortifiedvegetable oil produced (this is the simplest method). Alternatively, themetering pump should be calibrated by weekly testing and its in-line accuracyrecorded.2.3 Quality c<strong>on</strong>trol and feedback systems for implementingcorrective acti<strong>on</strong>sThe following quality c<strong>on</strong>trol procedures and feedback mechanisms were establishedas part of the quality c<strong>on</strong>trol m<strong>on</strong>itoring system developed for the oil fortificati<strong>on</strong>process:1. Product sampling and frequencyProcedure: Three to five samples of fortified vegetable oil (collected afterpackaging) should be taken daily from each producti<strong>on</strong> line and the levelsof vitamins A and D 3 measured. Levels should be <strong>with</strong>in 95–150% of thedeclared c<strong>on</strong>tent. One “composite sample” should be prepared daily fromeach producti<strong>on</strong> line and kept in an opaque airtight c<strong>on</strong>tainer for up to 3m<strong>on</strong>ths. These composite samples may be tested for their vitamin c<strong>on</strong>tentsby government inspectors. Four samples should be analysed m<strong>on</strong>thly by anexternal laboratory, and the results obtained used to verify the quality of theprocess.315
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS• Labelling of fortified vegetable oilsProcedure:Fortified vegetable oils must be identified <strong>with</strong> a label, which shouldspecify, as a mimimum, the product brand, the batch number, the address ofthe resp<strong>on</strong>sible entity, the date of producti<strong>on</strong> and durability, as well as thedeclared levels of vitamins A and D 3 .Fortified vegetable oils should be designatedusing the product’s usual name followed by the words “vitamins A &D 3 fortified”, or “vitamins A & D 3 enriched”. Any expressi<strong>on</strong> of a therapeuticnature of the product <strong>on</strong> the labels is not allowed, but functi<strong>on</strong>al nutriti<strong>on</strong>alallegati<strong>on</strong>s for the vitamins A and D 3 are permitted.• Distributi<strong>on</strong> of fortified vegetable oilsProcedure: Producers should be required to keep detailed records about thequantities of fortified oils they distribute to wholesalers and retailers. This isto facilitate the m<strong>on</strong>itoring of the turnover of fortified oils and the assuranceof the declared levels of vitamins A and D 3 .Every 3 m<strong>on</strong>ths, about 10 samplesshould be taken from retailers and households for testing. Whenever deviati<strong>on</strong>sfrom the admitted tolerances in vitamin A and D 3 c<strong>on</strong>tents are observed(−30% to +50%), an internal technical audit should be carried out to determinethe cause(s) of such deviati<strong>on</strong>s.• Documentati<strong>on</strong>Procedure: All results of quality assurance activities should be recorded andmade available to government inspectors up<strong>on</strong> request. A recall procedureshould be established to deal <strong>with</strong> cases of overdosed vegetable oils (i.e. thosec<strong>on</strong>taining high amounts of vitamins A and D 3 ) that might pose a threat toc<strong>on</strong>sumer health.• Inspecti<strong>on</strong> and technical auditsProcedure:Technical auditing, rather than sample testing, forms the mainstayof the inspecti<strong>on</strong> activities. At the factory level government inspecti<strong>on</strong> activitiesshould c<strong>on</strong>centrate <strong>on</strong> the internal quality c<strong>on</strong>trol and assurance proceduresadopted by individual manufacturers of fortified vegetable oils. Duevigilance must be given to corrective measures taken by producers to solveany limitati<strong>on</strong>s or errors. Attenti<strong>on</strong> should also be paid to the producti<strong>on</strong>equipment, c<strong>on</strong>diti<strong>on</strong>s of the premix storage and additi<strong>on</strong>, analysis andlabelling of fortified vegetable oils, and product storage c<strong>on</strong>diti<strong>on</strong>s. Warningsmust be issued to manufacturers in cases of negligence and deviati<strong>on</strong>s fromthe established procedures. If no corrective measures are taken by manufacturersto ensure compliance, an external technical audit should then be carriedout.— During each visit, between three and five samples of packaged productshould be taken and sent to the Official Laboratory of Analysis and316
E. A QUALITY CONTROL MONITORING SYSTEM FOR FORTIFIED VEGETABLE OILSChemical Research (OLACR) in Casablanca for analysis. Vitamin A andD 3 c<strong>on</strong>tents should lie between 95% and 150% of the declared levels.At the level of the wholesaler and retailer, inspecti<strong>on</strong> activities are mainlyc<strong>on</strong>cerned <strong>with</strong> labelling, turnover of fortified oils according to the“FIFO” (first in-first out) principle, and the c<strong>on</strong>diti<strong>on</strong>s of storage and handlingof these products.• Training activitiesProcedure: One-day training sessi<strong>on</strong>s should be scheduled for fortified oil producti<strong>on</strong>managers and government inspectors. The areas that should becovered during these sessi<strong>on</strong>s are as follows: techniques of vegetable oil refining;methods for vitamin A and D 3 analysis; techniques of vegetable oil sampling;factors affecting the stability of vitamins A and D 3 in vegetable oils; andthe principles of the HACCP approach and its applicati<strong>on</strong> to fortified vegetableoils.Reference1. Hazard analysis and critical c<strong>on</strong>trol point (HACCP) system and guidelines for itsapplicati<strong>on</strong>. Codex Alimentarius -Food hygiene- Basis texts- Sec<strong>on</strong>d editi<strong>on</strong>s. Rome,Food and Agriculture Organizati<strong>on</strong> of the United Nati<strong>on</strong>s, 1997: Annex.317
ANNEX FThe Codex Alimentarius and the WorldTrade Organizati<strong>on</strong> Agreements1. The Codex AlimentariusThe Codex Alimentarius, which means “<strong>food</strong> law” or “code” in Latin, is a comprehensivecollecti<strong>on</strong> of internati<strong>on</strong>ally adopted and uniformly presented <strong>food</strong>standards and related texts (including guidelines) that are comm<strong>on</strong>ly referredto as the “Codex texts”.The Codex texts address a wide range of general mattersthat apply to all processed, semi-processed and raw <strong>food</strong>s distributed to c<strong>on</strong>sumers,such as <strong>food</strong> hygiene, <strong>food</strong> additives, pesticide residues, c<strong>on</strong>taminants,labelling and presentati<strong>on</strong>, and methods of analysis and sampling. The texts alsodeal <strong>with</strong> various matters that are specific to individual commodities; forinstance, commodity standards, guidelines and related texts have been developedfor commodity groups such as milk, meat, cereals, and <strong>food</strong>s for specialdietary uses. The complete Codex Alimentarius is available via the Codex website 1 .The <strong>on</strong>going revisi<strong>on</strong> and development of the Codex Alimentarius is theresp<strong>on</strong>sibility of the Codex Alimentarius Commissi<strong>on</strong>, which was established inthe early 1963 as an intergovernmental body by the Food and Agriculture Organizati<strong>on</strong>of the United Nati<strong>on</strong>s (FAO) and the World Health Organizati<strong>on</strong>(WHO). Membership is open to all Member countries of FAO and/or WHO.The Codex texts are developed or revised though 29 subsidiary bodies comprisingregi<strong>on</strong>al, commodity and general committees, all of which are intergovernmentalin nature and most of which are currently active. The committees ofmost relevance to fortificati<strong>on</strong> and related issues are the Codex Committee <strong>on</strong>Nutriti<strong>on</strong> and Foods for Special Dietary Uses (CCNFSDU), which is hostedby Germany, and the Codex Committee <strong>on</strong> Food Labelling (CCFL), hosted byCanada.The terms of reference for the CCNFSDU is to advise <strong>on</strong> general nutriti<strong>on</strong>issues and to draft general provisi<strong>on</strong>s c<strong>on</strong>cerning the nutriti<strong>on</strong>al aspects ofall <strong>food</strong>s, develop standards, guidelines and related texts for <strong>food</strong>s for specialdietary uses (1). The remit of the Codex Committee <strong>on</strong> Food Labelling is tostudy problems related to the labelling and advertising of <strong>food</strong>s, to draft provisi<strong>on</strong>s<strong>on</strong> labelling that are applicable to all <strong>food</strong>s and to endorse draft provisi<strong>on</strong>s<strong>on</strong> labelling prepared by other Codex Committees.1www.codexalimentarius.net.318
F. THE CODEX ALIMENTARIUS AND THE WORLD TRADE ORGANIZATION AGREEMENTS1.1 Codex texts relevant to <strong>food</strong> fortificati<strong>on</strong>The part of the Codex Alimentarius of greatest direct relevance to <strong>food</strong> fortificati<strong>on</strong>is the General Principles for the Additi<strong>on</strong> of Essential Nutrients to Foods(CAC/GL 07-1987, amended 1989, 1991) (2). This secti<strong>on</strong>, which covers theadditi<strong>on</strong> of essential nutrients for the purposes of restorati<strong>on</strong>, nutriti<strong>on</strong>al equivalenceof substitute <strong>food</strong>s as well as fortificati<strong>on</strong>, provides guidance to governments<strong>with</strong> regard to the planning and implementati<strong>on</strong> of nati<strong>on</strong>al <strong>food</strong>fortificati<strong>on</strong> programmes.More specifically, the Codex General Principles for the Additi<strong>on</strong> of EssentialNutrients to Foods by:— providing guidance to those resp<strong>on</strong>sible for developing guidelines andlegal texts pertaining to the additi<strong>on</strong> of essential nutrients to <strong>food</strong>s; andby— establishing a uniform set of principles for the rati<strong>on</strong>al additi<strong>on</strong> of essentialnutrients to <strong>food</strong>s;seek to:— maintain or improve the overall nutriti<strong>on</strong>al quality of <strong>food</strong>s;— prevent the indiscriminate additi<strong>on</strong> of essential nutrients to <strong>food</strong>s, therebydecreasing the risk of health hazard due to essential nutrient excesses,deficits or imbalances (this also helps to prevent practices that may misleador deceive the c<strong>on</strong>sumer);— facilitate acceptance in internati<strong>on</strong>al trade of <strong>food</strong>s that c<strong>on</strong>tain addedessential nutrients.The General Principles state that the essential nutrient:• should be present at a level that will not result in an excessive or an insignificantintake of the added nutrient c<strong>on</strong>sidering the amounts from other sourcesin the diet;• should not result in an adverse effect <strong>on</strong> the metabolism of any othernutrient;• should be sufficiently stable in the <strong>food</strong> during packaging, storage, distributi<strong>on</strong>and use;• should be biologically available from the <strong>food</strong>;• should not impart undesirable characteristics to the <strong>food</strong>, or unduly shortenits shelf-life;319
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS• the additi<strong>on</strong>al cost should be reas<strong>on</strong>able for the intended c<strong>on</strong>sumers, and theadditi<strong>on</strong> of nutrients should not be used to mislead the c<strong>on</strong>sumer c<strong>on</strong>cerningthe nutriti<strong>on</strong>al quality of the <strong>food</strong>;• adequate technology and processing facilities should be available, as shouldmethods of measuring and/or enforcing the levels of added nutrients.A number of other Codex texts provide guidance and recommendati<strong>on</strong>s that areof relevance to fortified <strong>food</strong>s. Advice relating to the nutriti<strong>on</strong>al quality of <strong>food</strong>sfor special dietary uses is c<strong>on</strong>tained in Codex Alimentarius,Volume 4 – Foods forspecial dietary uses (3). Food labelling, nutriti<strong>on</strong> labelling, and claims that can beused by governments to establish their nati<strong>on</strong>al regulati<strong>on</strong>s are covered in CodexAlimentarius – Food labelling – Complete texts (4).1.2 Recommended levels of nutrients in <strong>food</strong>s for special dietary usesA series of Codex standards propose maximum and minimum levels of selectednutrients, in particular minerals and vitamins, for various <strong>food</strong>s having specialdietary uses, for example, <strong>food</strong>s for infants and children. Recommendedminimum and maximum vitamin and mineral levels for infant formulas are givenin the Codex Standard for Infant Formula (CODEX STAN 72-1981, amended1997) (5), and for follow-up formulas in the Codex Standard for Follow-upFormula (CODEX STAN 156-1987, amended 1989) (6). Rather than prescribingminimum and maximum nutrient levels, the Codex Standard forCanned Baby Foods (CAC/STAN 73-1981, amended 1989) (7) prefers to leavethis matter to the nati<strong>on</strong>al regulati<strong>on</strong>s of the country in which the <strong>food</strong> is sold.The Advisory List of Mineral Salts and Vitamin Compounds for Use in Foodsfor Infants and Children (CAC/GL 10-1979, amended 1991) (8) sets out recommendati<strong>on</strong>sregarding the source of any added minerals, their purity requirements,and the type of <strong>food</strong>s in which they can be used. In the case of thevitamins, the various forms are listed (<strong>with</strong> purity requirements), together <strong>with</strong>a number of specially formulated vitamin preparati<strong>on</strong>s, where applicable.The <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Formulated Supplementary Foods for Older Infants andYoung Children (CAC/GL 08-1991) not <strong>on</strong>ly provide recommendati<strong>on</strong>s relatingto nutriti<strong>on</strong>al matters but also address technical aspects of the producti<strong>on</strong>of formulated supplementary <strong>food</strong>s (9). These <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> include a list of referencedaily requirements for those vitamins and minerals “for which deficiencyis most frequently found in the diets of older infants and young children”, thesebeing the nutrients which should be given primary c<strong>on</strong>siderati<strong>on</strong> in the formulati<strong>on</strong>of supplementary <strong>food</strong>s. However, local c<strong>on</strong>diti<strong>on</strong>s, in particular, thenutrient c<strong>on</strong>tributi<strong>on</strong> of locally produced staple <strong>food</strong>s to the diet and thenutriti<strong>on</strong>al status of the target populati<strong>on</strong>, should be taken into account whendeciding which micr<strong>on</strong>utrients to add. The <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> make the general320
F. THE CODEX ALIMENTARIUS AND THE WORLD TRADE ORGANIZATION AGREEMENTSrecommendati<strong>on</strong> that when a <strong>food</strong> is supplemented <strong>with</strong> <strong>on</strong>e or more of the followingnutrients (vitamins A, D, E or C, thiamine (vitamin B 1 ), riboflavin(vitamin B 2 ), niacin, B 6 , folate, B 12 , calcium, ir<strong>on</strong>, iodine or zinc), the totalamount added per 100 g of dry <strong>food</strong> should be at least two thirds of the referencedaily requirement for that nutrient (9).In the Codex Standard for Processed Cereal-based Foods for Infants andChildren (CODEX STAN 74-1981, amended 1991) (10) maximum levels ofsodium are defined for different types of products covered by the standard. Itis also specified that “the additi<strong>on</strong> of vitamins, minerals and iodized salt shall bein c<strong>on</strong>formity <strong>with</strong> the legislati<strong>on</strong> of the country in which the product is sold”.1.3 LabellingGeneral labelling requirements are defined in the Codex General Standard forthe Labelling of Prepackaged Foods (CODEX STAN 01-1985, amended 2001)(11) and the Codex General <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Claims (CAC/GL 01-1979, revised1991) (12). Nutriti<strong>on</strong>al labelling is covered by the Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong>Labelling (CAC/GL 02-1985, revised 1993) (13) and nutriti<strong>on</strong>al claims bythe <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong> Claims (CAC/GL 23-1997, amended 2001)(14).The Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong> Labelling are based <strong>on</strong> the principle thatno <strong>food</strong> should be described or presented in a manner that is false, misleadingor deceptive, and that any claims made should be substantiated (13). A nutrientdeclarati<strong>on</strong>, defined in secti<strong>on</strong> 2.3 of the Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> as “a standardstatement or listing of the nutrient c<strong>on</strong>tent of a <strong>food</strong>”, is mandatory <strong>on</strong>ly whenclaims are made. The <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> include provisi<strong>on</strong>s for nutrient declarati<strong>on</strong>s,calculati<strong>on</strong> and presentati<strong>on</strong>. Nutrient Reference Values (NRVs) for labellingpurposes are defined for 14 vitamins and minerals, as well as for protein.The Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong> Claims (14) were developed asa supplement to the general provisi<strong>on</strong>s of the General <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Claims(12), primarily to provide a basis for the harm<strong>on</strong>izati<strong>on</strong> of nutriti<strong>on</strong> claims.Nutriti<strong>on</strong> claims are widely used as a marketing tool but have the potential tocause c<strong>on</strong>fusi<strong>on</strong> for c<strong>on</strong>sumers. The Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong>Claims specify that nutriti<strong>on</strong> claims must be c<strong>on</strong>sistent <strong>with</strong>, and support,nati<strong>on</strong>al nutriti<strong>on</strong> policy. Nutriti<strong>on</strong> claims that did not support nati<strong>on</strong>al policyshould not be permitted.The Codex texts recognize the importance of establishing a link betweennutriti<strong>on</strong> labelling provisi<strong>on</strong>s and nutriti<strong>on</strong> policy as a whole. Thus the Codextexts <strong>on</strong> nutriti<strong>on</strong> and labelling, by providing guidance to nati<strong>on</strong>al governments,allow for the development of nati<strong>on</strong>al regulati<strong>on</strong>s and requirements accordingto the specific needs of the populati<strong>on</strong>. C<strong>on</strong>diti<strong>on</strong>s have been defined for <strong>food</strong>sthat are a “source” of, or are “high” in, vitamins and minerals and protein.These321
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSprovisi<strong>on</strong>s apply to claims that are made about any <strong>food</strong>s, not just fortified <strong>food</strong>s.When such claims are made, nutrient declarati<strong>on</strong> should be provided in accordance<strong>with</strong> the <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong> Labelling (13), as menti<strong>on</strong>ed above.C<strong>on</strong>diti<strong>on</strong>s for the use of health claims are currently under discussi<strong>on</strong> by theCommissi<strong>on</strong>.2. The World Trade Organizati<strong>on</strong> AgreementsThe World Trade Organizati<strong>on</strong> (WTO) is the <strong>on</strong>ly internati<strong>on</strong>al organizati<strong>on</strong> inexistence that deals <strong>with</strong> the global rules of trade between nati<strong>on</strong>s. Its main functi<strong>on</strong>is to ensure that trade flows as smoothly, predictably and as freely as possible(15). By February 2002, 144 countries, which are collectively resp<strong>on</strong>siblefor more than 90% of world trade, had negotiated their accessi<strong>on</strong> to membershipof the WTO (16). Further informati<strong>on</strong> about the work of WTO and itsagreements is available via the WTO web site 1 .The two WTO agreements (17) of most relevance to <strong>food</strong> are the Agreement<strong>on</strong> the Applicati<strong>on</strong> of Sanitary and Phytosanitary Measures (the SPS Agreement),and the Agreement <strong>on</strong> Technical Barriers to Trade (the TBT Agreement).Under the terms of both agreements, countries may adopt provisi<strong>on</strong>s that limittrade for legitimate reas<strong>on</strong>s; the legitimate reas<strong>on</strong>s can include health c<strong>on</strong>siderati<strong>on</strong>s,provided that such measures do not unnecessarily restrict trade.However, it is the latter, the TBT Agreement, that usually has the more significantimplicati<strong>on</strong>s for <strong>food</strong> fortificati<strong>on</strong> regulati<strong>on</strong>s, whether mandatory and voluntary,and for this reas<strong>on</strong> is the focus of the discussi<strong>on</strong> here 2 .2.1 The Agreement <strong>on</strong> Technical Barriers to Trade: background andgeneral provisi<strong>on</strong>sIn the 1970s, C<strong>on</strong>tracting Parties to the General Agreement <strong>on</strong> Tariffs and Trade(GATT) expressed their dissatisfacti<strong>on</strong> <strong>with</strong> the emergence of new n<strong>on</strong>-tariffbarriers (NTBs) to trade. A GATT working group was thus established to evaluatethe impact of NTBs <strong>on</strong> internati<strong>on</strong>al trade, and reached the c<strong>on</strong>clusi<strong>on</strong> thatthe main form of NTBs that exporters faced were in fact technical barriers.During the Tokyo Round of GATT talks held in 1979 an Agreement <strong>on</strong> TechnicalBarriers to Trade (also called the Standards Code) which governed thepreparati<strong>on</strong>, adopti<strong>on</strong> and applicati<strong>on</strong> of technical regulati<strong>on</strong>s, standards andc<strong>on</strong>formity assessment procedures was drafted. The final form of the TBTAgreement was negotiated during the Uruguay Round in 1994 and entered intoforce in 1995, at the same time as the WTO.1www.wto.org.2This part of the <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> has been drafted by the WTO’s Trade and Envir<strong>on</strong>ment Divisi<strong>on</strong> andremains their resp<strong>on</strong>sibility.322
F. THE CODEX ALIMENTARIUS AND THE WORLD TRADE ORGANIZATION AGREEMENTSThe TBT Agreement is premised <strong>on</strong> an acknowledgement of the right ofWTO Members to develop technical requirements 1 , and to ensure that they arecomplied <strong>with</strong> (through what are known as c<strong>on</strong>formity assessment procedures).However, the objective of the TBT Agreement is to ensure that unnecessaryobstacles to internati<strong>on</strong>al trade are not created. This is achieved through anumber of principles that govern the preparati<strong>on</strong>, adopti<strong>on</strong> and applicati<strong>on</strong> ofmandatory and voluntary requirements and c<strong>on</strong>formity assessment procedures.These principles include:• n<strong>on</strong>-discriminati<strong>on</strong>;• the avoidance of unnecessary obstacles to internati<strong>on</strong>al trade;• harm<strong>on</strong>izati<strong>on</strong>;• the equivalence of technical regulati<strong>on</strong>s and of the results of c<strong>on</strong>formityassessment procedures;• mutual recogniti<strong>on</strong> of c<strong>on</strong>formity assessment procedures;• transparency.At the internati<strong>on</strong>al level, the TBT Agreement acts as an important instrumentto guard against the improper use of technical requirements and c<strong>on</strong>formityassessment procedures, that is to say, as disguised forms of restricti<strong>on</strong>s <strong>on</strong> trade.It also guards against the development of inefficient requirements and proceduresthat create avoidable obstacles to trade. In some settings, it can act as amechanism for encouraging countries to adopt less trade restrictive approachesto meeting regulatory objectives.2.2 Coverage and definiti<strong>on</strong>s of the TBT AgreementThe TBT Agreement divides technical requirements into three categories,namely technical regulati<strong>on</strong>s, standards and c<strong>on</strong>formity assessment procedures,which are defined as follows.• A technical regulati<strong>on</strong>: a “Document which lays down product characteristicsor their related processes and producti<strong>on</strong> methods, including the applicableadministrative provisi<strong>on</strong>s, <strong>with</strong> which compliance is mandatory. It may alsoinclude or deal exclusively <strong>with</strong> terminology, symbols, packaging, marking orlabelling requirements as they apply to a product, process or producti<strong>on</strong>method.”1The term “technical requirement” in the c<strong>on</strong>text of these <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> embraces both voluntaryand mandatory product specificati<strong>on</strong>s.323
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS• A standard: a “Document approved by a recognized body, that provides forcomm<strong>on</strong> and repeated use, rules, guidelines or characteristics for products orrelated processes and producti<strong>on</strong> methods, <strong>with</strong> which compliance is notmandatory. It may also include or deal exclusively <strong>with</strong> terminology, symbols,packaging, marking or labelling requirements as they apply to a product,process or producti<strong>on</strong> method.”• A c<strong>on</strong>formity assessment procedure: “Any procedure used, directly or indirectly,to determine that relevant requirements in technical regulati<strong>on</strong>s or standardsare fulfilled.”While both technical regulati<strong>on</strong>s and standards are technical product requirements,the main difference between the two is that compliance <strong>with</strong> technicalregulati<strong>on</strong>s is mandatory, whereas compliance <strong>with</strong> standards is voluntary. A lawthat stipulated that a nominated <strong>food</strong> must c<strong>on</strong>tain a minimum amount of amicr<strong>on</strong>utrient (as is the case <strong>with</strong> mandatory fortificati<strong>on</strong>) is an example of atechnical regulati<strong>on</strong>. Voluntary fortificati<strong>on</strong> provisi<strong>on</strong>s or a labelling permissi<strong>on</strong>for voluntary micr<strong>on</strong>utrient c<strong>on</strong>tent claims are examples of standards.The TBT Agreement c<strong>on</strong>tains provisi<strong>on</strong>s which ensure that technical regulati<strong>on</strong>sdo not act as unnecessary obstacles to trade. These provisi<strong>on</strong>s apply totechnical regulati<strong>on</strong>s developed by central and local governments, as well asthose developed by n<strong>on</strong>governmental bodies. WTO Members are fully resp<strong>on</strong>siblefor ensuring the observance of all the provisi<strong>on</strong>s of the TBT Agreement asthey relate to technical regulati<strong>on</strong>s. They must also formulate and implementpositive measures and mechanisms in support of the observance of the provisi<strong>on</strong>sof the TBT Agreement by local and n<strong>on</strong>governmental bodies.Standards are addressed separately under a “Code of Good Practice”, whichis c<strong>on</strong>tained in Annex 3 of the TBT Agreement. Most of the principles thatapply to technical regulati<strong>on</strong>s, also apply to standards through the Code. TheCode is open to acceptance by central, local and n<strong>on</strong>governmental standardizingbodies (at the nati<strong>on</strong>al level), as well as by regi<strong>on</strong>al governmental and n<strong>on</strong>governmentalbodies. However, the TBT Agreement notes that, “The obligati<strong>on</strong>sof Members <strong>with</strong> respect to compliance of standardizing bodies <strong>with</strong> the provisi<strong>on</strong>sof the Code of Good Practice shall apply irrespective of whether or not astandardizing body has accepted the Code of Good Practice.”C<strong>on</strong>formity assessment procedures are subject to many of the same principlesas those that apply to technical regulati<strong>on</strong>s and standards, in order to ensurethat they themselves do not c<strong>on</strong>stitute unnecessary obstacles to internati<strong>on</strong>altrade. WTO Members are fully resp<strong>on</strong>sible for ensuring observance of all provisi<strong>on</strong>srelating to c<strong>on</strong>formity assessment under the terms of the TBT Agreement,and must formulate and implement positive measures and mechanismsin support of the observance of the provisi<strong>on</strong>s by local government bodies. Theymust also ensure that central government bodies rely <strong>on</strong> c<strong>on</strong>formity assessment324
F. THE CODEX ALIMENTARIUS AND THE WORLD TRADE ORGANIZATION AGREEMENTSprocedures operated by n<strong>on</strong>governmental bodies, but <strong>on</strong>ly if such bodies are incompliance <strong>with</strong> the relevant provisi<strong>on</strong>s of the TBT Agreement.2.3 Legitimate objectivesUnder the TBT Agreement, technical regulati<strong>on</strong>s may be developed for <strong>on</strong>e ormore of the objectives c<strong>on</strong>sidered as legitimate by the TBT Agreement. Legitimateobjectives include: “inter alia, nati<strong>on</strong>al security requirements, the preventi<strong>on</strong>of deceptive practices, the protecti<strong>on</strong> of human health or safety, animal orplant life or health, or the envir<strong>on</strong>ment”. Fortificati<strong>on</strong> measures are most likelyto fall under the protecti<strong>on</strong> of human health category. However, the preventi<strong>on</strong>of deceptive practices, which refers to measures that mislead or deceive c<strong>on</strong>sumers(e.g. false nutriti<strong>on</strong>al informati<strong>on</strong> given <strong>on</strong> <strong>food</strong> labels), might also c<strong>on</strong>stitutea legitimate objective and thus WTO Members would be allowed to adopttechnical regulati<strong>on</strong>s to guard against such practices.The risks associated <strong>with</strong> legitimate objectives are assessed against a numberof factors, including: “inter alia, available scientific and technical informati<strong>on</strong>,related processing technology or intended end-uses of products”. Once again,the inclusi<strong>on</strong> of the words “inter alia”, indicates that some flexibility may beexercised in the selecti<strong>on</strong> of factors against which risks may be assessed.2.4 Principles which govern the preparati<strong>on</strong>, adopti<strong>on</strong> and applicati<strong>on</strong> ofmandatory and voluntary requirements and c<strong>on</strong>formity assessmentprocedures2.4.1 N<strong>on</strong>-discriminati<strong>on</strong>The principle of n<strong>on</strong>-discriminati<strong>on</strong> forms the backb<strong>on</strong>e of the internati<strong>on</strong>altrading system. The TBT Agreement embraces the GATT principle of n<strong>on</strong>discriminati<strong>on</strong>,and applies it to technical regulati<strong>on</strong>s, standards and c<strong>on</strong>formityassessment procedures. In general, it is the principle that outlaws discriminati<strong>on</strong>between products of WTO Member countries, and between imported anddomestically produced products.With respect to both technical regulati<strong>on</strong>s and standards, the TBT Agreementstipulates that the n<strong>on</strong>-discriminati<strong>on</strong> principle be observed throughout thevarious stages of their preparati<strong>on</strong>, adopti<strong>on</strong> and applicati<strong>on</strong>. For instance, aWTO Member cannot adopt a technical regulati<strong>on</strong> mandating that all imported<strong>food</strong> meet certain micr<strong>on</strong>utrient standards, if it does not enforce such standards<strong>on</strong> its own domestically produced <strong>food</strong>. Nor can it enforce a technical regulati<strong>on</strong><strong>on</strong> <strong>on</strong>e, but not <strong>on</strong> another, of its trading partners. In short, under the disciplinesof the TBT Agreement and the WTO system as a whole, treatment mustbe no less favourable.WTO Members must also ensure that c<strong>on</strong>formity assessment proceduresare not prepared, adopted or applied in a discriminatory manner. Achieving325
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSn<strong>on</strong>-discriminati<strong>on</strong> <strong>with</strong> respect to c<strong>on</strong>formity assessment requires, am<strong>on</strong>g otherthings, ensuring suppliers’ right to c<strong>on</strong>formity assessment under the rules of procedure,including the opti<strong>on</strong> of having c<strong>on</strong>formity assessment activities undertakenin situ and to receive the mark of the system. C<strong>on</strong>formity assessmentsystems must not distinguish between the procedures to be followed for productsoriginating from different sources. For instance, systems cannot subjectsimilar products to tests of varying degrees of stringency depending <strong>on</strong> theirsource of supply.2.4.2 Avoidance of unnecessary obstacles to internati<strong>on</strong>al tradeThe avoidance of unnecessary obstacles to internati<strong>on</strong>al trade is the principalobjective of the TBT Agreement. With respect to both technical regulati<strong>on</strong>s andstandards, the TBT Agreement states that WTO Members must ensure thatneither technical regulati<strong>on</strong>s nor standards are “prepared, adopted or applied<strong>with</strong> a view to or <strong>with</strong> the effect of creating unnecessary obstacles to internati<strong>on</strong>altrade”. With respect to technical regulati<strong>on</strong>s, the TBT Agreement elaborates<strong>on</strong> the meaning of this phrase; it stipulates that technical regulati<strong>on</strong>s maynot be more trade restrictive than is necessary to fulfil a legitimate objective,taking into account the risks that n<strong>on</strong>-fulfilment would create.Determining whether or not a technical regulati<strong>on</strong> poses an unnecessary obstacleto internati<strong>on</strong>al trade involves two steps. Firstly, the regulati<strong>on</strong> mustbe designed to meet <strong>on</strong>e of the legitimate objectives delineated in the TBTAgreement (see secti<strong>on</strong> 2.3). Sec<strong>on</strong>dly, the regulati<strong>on</strong> must be the least traderestrictiveopti<strong>on</strong> available to a WTO Member that achieves that legitimate objective,taking into account the risks that would be associated <strong>with</strong> its n<strong>on</strong>-fulfilment.The TBT Agreement encourages WTO Members to develop technical regulati<strong>on</strong>sand standards that are based <strong>on</strong> product performance requirements,rather than <strong>on</strong> design requirements. The former creates fewer obstacles to trade,providing exporters greater leeway in terms of fulfilling the objectives of thetechnical requirements. For instance, it would be preferable for a country to stipulatethe minimum amount of a micr<strong>on</strong>utrient that must be present in a specifictype of <strong>food</strong> rather than a specific process for the additi<strong>on</strong> of thatmicr<strong>on</strong>utrient.To help avoid unnecessary obstacles to internati<strong>on</strong>al trade, the TBT Agreementrequires WTO Members to revoke technical regulati<strong>on</strong>s when the objectivesthat had given rise to their adopti<strong>on</strong> no l<strong>on</strong>ger exist, or if changedcircumstances or objectives can be addressed in a less trade-restrictive manner.WTO Members must also ensure that unnecessary obstacles to internati<strong>on</strong>altrade are avoided when preparing, adopting and applying c<strong>on</strong>formity assessmentprocedures for technical regulati<strong>on</strong>s and standards. The TBT Agreementstates that, “C<strong>on</strong>formity assessment procedures shall not be more strict or be326
F. THE CODEX ALIMENTARIUS AND THE WORLD TRADE ORGANIZATION AGREEMENTSapplied more strictly than is necessary to give the importing Member adequatec<strong>on</strong>fidence that products c<strong>on</strong>form <strong>with</strong> the applicable technical regulati<strong>on</strong>s orstandards, taking account of the risks that n<strong>on</strong>-c<strong>on</strong>formity would create.” Inother words, c<strong>on</strong>formity assessment procedures must not be applied more stringentlythan is necessary to ensure c<strong>on</strong>formity. They must c<strong>on</strong>sider the risks ofreduced stringency, and decide whether or not the risks outweigh the benefitsof having fewer obstacles to internati<strong>on</strong>al trade.The TBT Agreement also urges Members to ensure that c<strong>on</strong>formity assessmentprocedures are undertaken as expeditiously as possible, that informati<strong>on</strong>requirements are limited to whatever is necessary, that the c<strong>on</strong>fidentiality ofinformati<strong>on</strong> is respected for legitimate commercial interests, and finally that thefees charged domestically are equitable to the fees charged for foreign products.2.4.3 Harm<strong>on</strong>izati<strong>on</strong>The TBT Agreement encourages WTO Members to base their technical regulati<strong>on</strong>s,standards and c<strong>on</strong>formity assessment procedures <strong>on</strong> internati<strong>on</strong>al standards,guidelines and recommendati<strong>on</strong>s, when these exist or their completi<strong>on</strong> isimminent, excepting when they are deemed to be inappropriate or ineffective.For example, it allows derogati<strong>on</strong> from technical regulati<strong>on</strong>s and standards inthe event of climatic or geographic differences, or because of fundamental technologicalproblems. Although not specifically refered to in the TBT Agreement,the Codex Alimentarius is widely interpreted as being the relevant text or “goldstandard” <strong>with</strong> respect to the development of regulati<strong>on</strong>s <strong>on</strong> <strong>food</strong> products.The call for harm<strong>on</strong>izati<strong>on</strong> is intended to avoid undue layers of technicalrequirements and assessment procedures, and to encourage the wider applicati<strong>on</strong>of those that have already been developed and approved by the internati<strong>on</strong>alcommunity. To support this endeavour, the TBT Agreement calls up<strong>on</strong>WTO Members to participate in the work of internati<strong>on</strong>al standardizing andc<strong>on</strong>formity assessment bodies.2.4.4 Equivalence and mutual recogniti<strong>on</strong>Internati<strong>on</strong>al harm<strong>on</strong>izati<strong>on</strong> is a time-c<strong>on</strong>suming process, and is sometimes difficultto achieve. The principle of equivalency is thus designed to complementthat of harm<strong>on</strong>izati<strong>on</strong> and the TBT Agreement encourages WTO Members toaccept each other’s regulati<strong>on</strong>s as equivalent until internati<strong>on</strong>al harm<strong>on</strong>izati<strong>on</strong>becomes possible. More specifically, the TBT Agreement stipulates that WTOMembers give positive c<strong>on</strong>siderati<strong>on</strong> to recognizing other Members’ technicalregulati<strong>on</strong>s as being equivalent to their own, even when they differ, providedthat they are satisfied that the regulati<strong>on</strong>s adequately fulfil their objective.Through the establishment of equivalency arrangements between countries,products that meet the regulati<strong>on</strong>s of the exporting country do not have to327
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTScomply <strong>with</strong> the regulati<strong>on</strong>s of the importing country, so l<strong>on</strong>g as the same objectivesare fulfilled by the two sets of requirements. This significantly reduces barriersto trade.The TBT Agreement also calls up<strong>on</strong> WTO Members to ensure, wheneverpossible, that the results of c<strong>on</strong>formity assessment procedures of other MemberCountries are accepted, even when they differ from their own, provided that theprocedures give the same level of c<strong>on</strong>fidence. The purpose of this provisi<strong>on</strong> isto avoid multiple product testing (in both exporting and importing countrymarkets), and its associated costs. However, it is acknowledged that in order toachieve acceptance, negotiati<strong>on</strong>s may be needed, primarily to ensure the c<strong>on</strong>tinuedreliability of c<strong>on</strong>formity assessment results (the accreditati<strong>on</strong> of c<strong>on</strong>formityassessment bodies is a factor that can be taken into account in thisregard). The TBT Agreement encourages these kinds of mutual recogniti<strong>on</strong>agreements between WTO Members.2.4.5 TransparencyTransparency is a central feature of the TBT Agreement, and is achievedthrough notificati<strong>on</strong> obligati<strong>on</strong>s, the establishment of enquiry points, and thecreati<strong>on</strong> of the WTO TBT Committee.Notificati<strong>on</strong> obligati<strong>on</strong>s require WTO Members to notify their draft technicalregulati<strong>on</strong>s, standards and c<strong>on</strong>formity assessment procedures and also toallow other Members sufficient time to comment <strong>on</strong> them. Members are obligedto take comments from other countries into account. 1 Notificati<strong>on</strong>s provide auseful means of disseminating informati<strong>on</strong>, and can often help to avoid unnecessaryobstacles to internati<strong>on</strong>al trade at an early stage. The advantage of thenotificati<strong>on</strong> system is that it provides exporters <strong>with</strong> the opportunity to learn ofnew requirements prior to their entry into force, to comment <strong>on</strong> these requirements(and know that their comments will be taken into account), and to preparethemselves for compliance.The TBT Agreement stipulates that each WTO Member establish an enquirypoint for resp<strong>on</strong>ding to questi<strong>on</strong>s <strong>on</strong> technical regulati<strong>on</strong>s, standards and c<strong>on</strong>formityassessment procedures (whether proposed or adopted), and for supplyingrelevant documents.A TBT Committee has been established as part of the TBT Agreement toact as a forum for c<strong>on</strong>sultati<strong>on</strong> and negotiati<strong>on</strong> <strong>on</strong> all issues pertaining to theAgreement. Participati<strong>on</strong> in the Committee is open to all WTO Members, anda number of internati<strong>on</strong>al standardizing bodies are invited to attend meetings asobservers.1Draft technical regulati<strong>on</strong>s and c<strong>on</strong>formity assessment procedures <strong>on</strong>ly have to be notified whenan internati<strong>on</strong>al standard, guide or recommendati<strong>on</strong>, does not exist (or they are not in accordance<strong>with</strong> existing <strong>on</strong>es), and if they may a have a significant effect <strong>on</strong> the trade of other WTOMembers.328
F. THE CODEX ALIMENTARIUS AND THE WORLD TRADE ORGANIZATION AGREEMENTSReferences1. Codex Alimentarius Commissi<strong>on</strong>. Procedural manual. 12th ed. Rome, Food andAgriculture Organizati<strong>on</strong> of the United Nati<strong>on</strong>s, 2001.2. General Principles for the Additi<strong>on</strong> of Essential Nutrients to Foods CAC/GL 09-1987(amended 1989, 1991). Rome, Joint FAO/WHO Food Standards Programme, CodexAlimentarius Commisi<strong>on</strong>, 1987 (http://www.codexalimentarius.net/download/standards/299/CXG_009e.pdf, accessed 7 October 2005).3. Codex Alimentarius, Volume 4. Foods for special dietary uses. 2nd ed. Rome, JointFAO/WHO Food Standard Programme, Codex Alimentarius Commisi<strong>on</strong>, 1994.4. Codex Alimentarius – Food labelling – Complete texts. Rome, Food and AgricultureOrganizati<strong>on</strong> of the United Nati<strong>on</strong>s, 2001.5. Codex Alimentarius Commissi<strong>on</strong>. Codex Standard for Infant Formula CODEX STAN72-1981 (amended 1983, 1985, 1987, 1997). Joint FAO/WHO Food Standards Programme,Codex Alimentarius Commissi<strong>on</strong>, 1981 (http://www.codexalimentarius.net/download/standards/288/CXS_072e.pdf, accessed 7 October 2005).6. Codex Alimentarius Commissi<strong>on</strong>. Codex Standard Follow-up Formula CODEX STAN156-1987 (amended 1989). Joint FAO/WHO Food Standards Programme, CodexAlimentarius Commissi<strong>on</strong>, 1987 (http://www.codexalimentarius.net/download/standards/293/CXS_156e.pdf, accessed 7 October 2005).7. Codex Alimentarius Commissi<strong>on</strong>. Codex Standard for Canned Baby Foods CODEXSTAN 73-1981 (amended 1985, 1987, 1989). Joint FAO/WHO Food Standards Programme,Codex Alimentarius Commissi<strong>on</strong>, 1981 (http://www.codexalimentarius.net/download/standards/289/CXS_073e.pdf, accessed 7 October 2005).8. Codex Alimentarius Commissi<strong>on</strong>. Advisory List of Minereal Salts and VitaminCompounds for Use in Foods for Infants and Children, CAC/GL 10-1979 (amended1983, 1991). Joint FAO/WHO Food Standards Programme, Codex AlimentariusCommissi<strong>on</strong>, 1979 (http://www.codexalimentarius.net/download/standards/300/CXG_010e.pdf, accessed 7 October 2005).9. <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Formulated Supplementary Foods for Older Infants and Young ChildrenCAC/GL 08-1991. Joint FAO/WHO Food Standards Programme, Codex AlimentariusCommisi<strong>on</strong>, 1991 (http://www.codexalimentarius.net/download/standards/298/CXG_008e.pdf, accessed 7 October 2005).10. Codex Alimentarius Commissi<strong>on</strong>. Codex Standard for Processed Cereal-based Foodsfor Infants and Children CODEX STAN 74-1981 (amended 1985, 1987, 1989, 1991).Joint FAO/WHO Food Standards Programme, Codex Alimentarius Commissi<strong>on</strong>,1981 (http://www.codexalimentarius.net/download/standards/290/CXS_074e.pdf,accessed 7 October 2005).11. Codex General Standard for the Labelling of Prepackaged Foods CODEX STAN 1-1985(revised 1985, 1991, 1999, 2001). Rome, Joint FAO/WHO Food Standard Programme,Codex Alimentarius Commisi<strong>on</strong>, 1985 (http://www.codexalimentarius.net/download/standards/32/CXS_001e.pdf, accessed 7th October 2005).12. Codex General <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Claims CAC/GL 01-1979, (revised 1991). JointFAO/WHO Food Standard Programme, Codex Alimentarius Commisi<strong>on</strong>, 1985(http://www.codexalimentarius.net/download/standards/33/CXG_001e.pdf,accessed 7 October 2005).13. Codex <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong> Labelling CAC/GL 02-1985, (revised 1993). JointFAO/WHO Food Standard Programme, Codex Alimentarius Commisi<strong>on</strong>, 1985(http://www.codexalimentarius.net/download/standards/34/CXG_002e.pdf,accessed 7 October 2005).329
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTS14. <str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong> Claims CAC/GL 23-1997, (revised 2004). JointFAO/WHO Food Standards Programme, Codex Alimentarius Commisi<strong>on</strong>, 1997(http://www.codexalimentarius.net/download/standards/351/CXG_023e.pdf,accessed 7 October 2005).15. The WTO in brief. Geneva, World Trade Organizati<strong>on</strong>, 2003 (http://www.wto.org/english/res_e/doload_e/10b_e.pdf, accessed 22/02/05).16. WTO agreements and public health: a joint study by the WHO and WTO secretariat.Geneva, World Health Organizati<strong>on</strong>, 2002.17. The results of the Uruguay Round of multilateral trade negotiati<strong>on</strong>s – the legaltexts. Geneva, World Trade Organizati<strong>on</strong>, 1995 (http://www.wto.org/english/docs_e/legal_e/legal_e.htm, accessed 19/04/05).330
IndexAdequate Intake (AI), 146Advocacy, 228policy-makers, 228Africafolate deficiency, 63inadequate iodine nutriti<strong>on</strong>,54iodine fortificati<strong>on</strong>, 121pellagra, 74vitamin A deficiency, 49vitamin C deficiency, 78vitamin C fortificati<strong>on</strong>, 130Africa, sub-Saharanir<strong>on</strong> deficiency, 43vitamin A fortificati<strong>on</strong>, 14Agreement <strong>on</strong> Technical Barriers toTrade. See TBT AgreementAgreement <strong>on</strong> the Applicati<strong>on</strong> ofSanitary and PhytosanitaryMeasures. See SPS AgreementAmericasinadequate iodine nutriti<strong>on</strong>,54Anaemia, 44prevalence, 44Appropriate nutrient compositi<strong>on</strong> of aspecial purpose <strong>food</strong>, 26Ascorbic acid. See also vitamin Cfortificantsir<strong>on</strong> fortificati<strong>on</strong>, 101Asiaiodine fortificati<strong>on</strong>, 121Australiamandatory fortificati<strong>on</strong>, 32Average Requirement (AR), 146Beriberi, 17, 20, 68, 71Biofortificati<strong>on</strong>, 30B<strong>on</strong>e mineral density (BMD), 85Botswanamultiple fortificati<strong>on</strong>, 17prevalence of vitamin B 12 deficiency,66Brazilir<strong>on</strong> fortificati<strong>on</strong>, 108Breadiodine fortificati<strong>on</strong>, 121Calciumdefined, 84Calcium deficiencyhealth c<strong>on</strong>sequences of, 86osteoporosis, 85prevalence of, 84rickets, 86risk factors for, 85Calcium fortificants, 131Calcium fortificati<strong>on</strong>defined, 131wheat flour, 131Calcium salts, 132Canadafolic acid fortificati<strong>on</strong>, 14, 128mass fortificati<strong>on</strong>, 27vitamin A fortificati<strong>on</strong>, 19, 113vitamin D fortificati<strong>on</strong>, 20Cancer. See ir<strong>on</strong> intakeCausality. See impactevaluati<strong>on</strong>:probability evaluati<strong>on</strong>Central African Republiciodine fortificati<strong>on</strong>, 121Central Americair<strong>on</strong> fortificati<strong>on</strong>, 99, 106vitamin A fortificati<strong>on</strong>, 14, 19Cerealsfolic acid fortificati<strong>on</strong>, 128zinc fortificati<strong>on</strong>, 125CCP. See critical c<strong>on</strong>trol points331
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSChildrenvitamin A deficiency, 5zinc deficiency, 5Chilefluoride fortificati<strong>on</strong>, 134folic acid fortificati<strong>on</strong>, 128ir<strong>on</strong> and vitamin C fortificati<strong>on</strong>, 17ir<strong>on</strong> fortificati<strong>on</strong>, 101, 106vitamin A fortificati<strong>on</strong>, 20vitamin C fortificati<strong>on</strong>, 81Chinacalcium deficiency, 86folic acid supplementati<strong>on</strong> trials,63iodine fortificati<strong>on</strong>, 122ir<strong>on</strong> fortificati<strong>on</strong>, 15, 109pellagra, 74riboflavin deficiency, 73selenium deficiency, 88selenium fortificati<strong>on</strong>, 89, 133vitamin D deficiency, 82Cobalamin. See vitamin B 12Cocoair<strong>on</strong> fortificati<strong>on</strong>, 109Codex Alimentarius Commissi<strong>on</strong>, 172,176, 318Advisory List of Mineral Salts andVitamin Compounds for Use inFoods for Infants and Children,320Committee <strong>on</strong> Food Labelling(CCFL), 318Committee <strong>on</strong> Nutriti<strong>on</strong> and Foodsfor Special Dietary Uses(CCNFSDU), 318General Principles for the Additi<strong>on</strong>of Essential Nutrients to Foods,319<str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> for Use of Nutriti<strong>on</strong>Claims, 321<str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> FormulatedSupplementary Foods for OlderInfants and Young Children,320<str<strong>on</strong>g>Guidelines</str<strong>on</strong>g> <strong>on</strong> Nutriti<strong>on</strong> Labelling,321Standard for Canned Baby Foods,320Standard for Infant Formula, 320Standard for Processed Cereal-basedFoods for Infants and Children,321Standard for the Labelling ofPrepackaged Foods, 321Complementary <strong>food</strong>sdefined, 107fortificati<strong>on</strong>, 169ir<strong>on</strong> fortificati<strong>on</strong>, 107zinc fortificati<strong>on</strong>, 125C<strong>on</strong>sumer marketing strategies, 238demand-driven, 238supply-driven, 238Cost calculati<strong>on</strong>cost of m<strong>on</strong>itoring and evaluati<strong>on</strong>,211industry costs (t), 212initial investment costs, 211recurrent costs, 211Costa Ricafluoride fortificati<strong>on</strong>, 134folic acid fortificati<strong>on</strong>, 128prevalence of vitamin B 12 deficiency,66Cost–benefit analysis, 210, 215Cost-effectivenessanalysis, 208cost per death averted, 207cost per disability-adjusted life-yearsaved, 207cost–benefit ratio, 210defined, 207, 223sensitivity analysis, 217Critical c<strong>on</strong>trol points (CCP),314–15Cubathiamine deficiency, 70Dairy productsir<strong>on</strong> fortificati<strong>on</strong>, 108DALYs. See disability-adjusted life yearsDental cariespreventi<strong>on</strong>, 134Dietary Reference Intakes (DRIs)Adequate Intake (AI), 146Average Requirement (AR), 146Estimated Average Requirement(EAR), 146Lower Reference Nutrient Intake, 146332
INDEXLower Threshold Intake, 146Recommended Dietary Allowance(RDA), 146Safe Intake, 146Tolerable Upper Level (UL), 146Disability-adjusted life years (DALYs),defined, 3Djiboutithiamine deficiency, 70Dominican Republicfolic acid fortificati<strong>on</strong>, 128Dual fortificati<strong>on</strong>salt, 18EAR cut-point method. See EstimatedAverage Requirement (EAR)Eastern Mediterraneaninadequate iodine nutriti<strong>on</strong>, 54prevalence of vitamin A deficiency,49Egyptvitamin B 6 deficiency, 76El Salvadorfolic acid fortificati<strong>on</strong>, 128vitamin A fortificati<strong>on</strong>, 116Enrichmentdefined, 25Erythrocyte protoporphyrinas indicator for ir<strong>on</strong> deficiency (t),46Estimated Average Requirement (EAR),146EAR cut-point method, 143Ethiopiathiamine deficiency, 70Europeinadequate iodine nutriti<strong>on</strong>, 54iodine fortificati<strong>on</strong>, 121pellagra, 74European Uni<strong>on</strong>market-driven fortificati<strong>on</strong>, 28voluntary fortificati<strong>on</strong>, 34Evaluati<strong>on</strong>impact evaluati<strong>on</strong>, 180Ferric phosphate compounds, 100Ferric pyrophosphatemicr<strong>on</strong>izing, 104Ferric saccharate, 99Ferritinas indicator for ir<strong>on</strong> deficiency (t),45Ferrous bisglycinate, 103Ferrous fumarate, 99encapsulated, 103Ferrous sulfate, 99encapsulated, 103Fertilizerselenium fortificati<strong>on</strong>, 133Finlandiodine fortificati<strong>on</strong>, 122selenium fortificati<strong>on</strong>, 89Flour Fortificati<strong>on</strong> Initiative,233Fluoridedefined, 89Fluoride deficiencydefined, 89health c<strong>on</strong>sequences of, 90risk factors for, 90Fluoride fortificants, 134Folatedefined, 61dietary folate equivalents (DFE),160intake levels, 160Pan American Health Organizati<strong>on</strong>(PAHO), 160Folate deficiencyhealth c<strong>on</strong>sequences of, 63prevalence of, 61–62risk factors for, 63Folic acid, 126Folic acid fortificants. See also vitamin Bfortificantssafety issues, 129Folic acid fortificati<strong>on</strong>cereals, 128efficacy trials, 19Food and Agriculture Organizati<strong>on</strong>(FAO), xviii, 41Food-based strategies, 11breastfeeding, 12dietary diversity, 12dietary quality, 12Food fortificantsdefined, 95iodine, 118333
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSFood fortificati<strong>on</strong>blended <strong>food</strong>s, 169complementary <strong>food</strong>s, 169c<strong>on</strong>sumer percepti<strong>on</strong>s, xixcost limit, 164cost-effectiveness. See costeffectivenessdefined, 13, 24–25efficacy trials, 15history of, 14human rights, xixmarket-driven, 171overview, xixproducti<strong>on</strong> costs, xixregulati<strong>on</strong> of, 31safety, 162–63, 212targeted, 169technological limit, 162–63, 212voluntary. See voluntary fortificati<strong>on</strong>Food fortificati<strong>on</strong> levelscost limit, 298Feasible Fortificati<strong>on</strong> Level (FFL),295Legal Minimum Level (LmL), 296Maximum Fortificati<strong>on</strong> Level(MFL), 296Maximum Tolerable Level (MTL),297Minimum Fortificati<strong>on</strong> Level (mFL),295safety limit, 297Target Fortificati<strong>on</strong> Level (TFL),295technological limit, 298Tolerable Upper Intake Level (UL),294Food fortificati<strong>on</strong> programmesadvocacy. See advocacybiomarker, 141commercial m<strong>on</strong>itoring, 182c<strong>on</strong>sumer marketing strategies. Seec<strong>on</strong>sumer marketing strategiescoverage methods, 195data collecti<strong>on</strong>, 178design and planning, 95, 178dietary goal, 142dietary intakes, 139dietary patterns, 139EAR cut-point method, 143evaluati<strong>on</strong>, 179external m<strong>on</strong>itoring, 180guidelines, see World FoodProgramme (WFP)household/individual m<strong>on</strong>itoring, 179impact evaluati<strong>on</strong>, 180. See impactevaluati<strong>on</strong>informati<strong>on</strong> needs, 139internal m<strong>on</strong>itoring, 180m<strong>on</strong>itoring and evaluating, 178nutriti<strong>on</strong>al status, 139operati<strong>on</strong>al performance, 178overview, xv, xviplanning (t), 140probability method, 143, 157promoti<strong>on</strong>. See promoti<strong>on</strong>regulatory m<strong>on</strong>itoring, 179skewed requirements, 157vitamin A, 113Food law, 241claims, nutriti<strong>on</strong> and health-related,249compositi<strong>on</strong>, 244c<strong>on</strong>tain, 245<strong>food</strong> labelling, 247–48legal minimum and maximum levels,245mandatory fortificati<strong>on</strong>, 243–44minimum and maximum levels, 254minimum claim criteria, 254name of <strong>food</strong>, 244name of micr<strong>on</strong>utrient, 246permitted fortificant compounds,247Philippines Act Promoting SaltIodizati<strong>on</strong> Nati<strong>on</strong>wide, 242voluntary fortificati<strong>on</strong>. See voluntaryfortificati<strong>on</strong>Food systemsoverview, xvFood vehiclesdefined, xix, 95fluoride, 134ir<strong>on</strong> fortificati<strong>on</strong>, 104limits <strong>on</strong> fortificants, 23salt, 110vitamin A, 111, 113Francevitamin D deficiency, 82334
INDEXGambia, Theberiberi, 71calcium supplementati<strong>on</strong>, 86riboflavin deficiency, 73thiamine deficiency, 70General Agreement <strong>on</strong> Tariffs and Trade(GATT), 322General Principles for the Additi<strong>on</strong> ofEssential Nutrients to Foods, 25Germanyprevalence of vitamin B 12 deficiency,66Global Alliance for Improved Nutriti<strong>on</strong>(GAIN), 11, 223Global trade agreements, 240SPS Agreement, 240TBT Agreement, 240World Trade Organizati<strong>on</strong> (WTO),240, 322Goitre, 54goitrogens, 54Guatemalafolic acid fortificati<strong>on</strong>, 128riboflavin deficiency, 73targeted fortificati<strong>on</strong>, 28vitamin A fortificati<strong>on</strong>, 19, 116vitamin B 12 deficiency, 67Guineathiamine deficiency, 70HACCP. See hazard analysis and criticalc<strong>on</strong>trol pointHaemoglobinas indicator for ir<strong>on</strong> deficiency (t), 45Hazard analysis and critical c<strong>on</strong>trolpoint (HACCP), 314Helicobacter pylori infecti<strong>on</strong>. Seevitamin B 12 deficiencyH<strong>on</strong>durasfolic acid fortificati<strong>on</strong>, 128vitamin A fortificati<strong>on</strong>, 116Household and community fortificati<strong>on</strong>,29Hungaryfluoride fortificati<strong>on</strong>, 134Impact evaluati<strong>on</strong>, 196adequacy evaluati<strong>on</strong>, 197outcome indicators, 200plausibility evaluati<strong>on</strong>, 197probability evaluati<strong>on</strong>, 199regulatory m<strong>on</strong>itoring, 204timing, 202Indiafolate deficiency, 63ir<strong>on</strong> deficiency, 43prevalence of vitamin B 12 deficiency,66vitamin D deficiency, 83zinc supplementati<strong>on</strong>, 61Ind<strong>on</strong>esiafolic acid fortificati<strong>on</strong>, 128targeted fortificati<strong>on</strong>, 28thiamine deficiency, 70zinc fortificati<strong>on</strong>, 125INFOODS, 153Intake24-hour recall survey, 152excessive, 151folate, 160inadequate, 151median nutrient, 150target median, 147, 150Internati<strong>on</strong>al C<strong>on</strong>ference <strong>on</strong> Nutriti<strong>on</strong>(ICN), xviiiInternati<strong>on</strong>al Resource Laboratory forIodine network (IRLI), 121Internati<strong>on</strong>al Zinc Nutriti<strong>on</strong>C<strong>on</strong>sultative Group (IZiNCG),124Iodate, 118Iodide, 118Iodinedefined, 52WHO fortificati<strong>on</strong> levels in salt,159Iodine deficiencycorrecti<strong>on</strong> of, 56Council for C<strong>on</strong>trol of IodineDeficiency Disorders (ICCIDD),287DALYs, 3disorders, 54, 55 (t)Global Network for SustainedEliminati<strong>on</strong> of Iodine Deficiency,The, 11, 222–23goitre, 52health c<strong>on</strong>sequences of, 54335
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSInternati<strong>on</strong>al Resource Laboratoryfor Iodine network (IRLI), The,287mental retardati<strong>on</strong>, 52, 55prevalence of, 52risk factors for, 54sustainable eliminati<strong>on</strong>, 285Iodine fortificati<strong>on</strong>bread, 121efficacy trials, 17ir<strong>on</strong>. See dual fortificati<strong>on</strong>safety issues, 122water, 121Iodine-induced hyperthyroidism (IIH),122–23Iodine-induced thyroiditis, 123Iodized salt. See saltIr<strong>on</strong>bioavailability from fortificants,100water-soluble forms, 98Ir<strong>on</strong> deficiencyanaemia, 44benefits of interventi<strong>on</strong>, 48DALYs, 3defined, 43health c<strong>on</strong>sequences of, 48mortality data, 3prevalence of, 43risk factors, 44transferrin. See transferrinIr<strong>on</strong> fortificants, 97alternative, 101sensory changes, 104Ir<strong>on</strong> fortificati<strong>on</strong>cocoa, 109complementary <strong>food</strong>s, 107curry powder, 16dairy products, 108efficacy trials, 15, 17encapsulati<strong>on</strong>, 110iodine. See dual fortificati<strong>on</strong>maize flours, 107rice, 108safety issues, 110salt, 110soy sauce, fish sauce, 109SUSTAIN Task Force, 106wheat flour, 105Ir<strong>on</strong> intakecancer, 110Ir<strong>on</strong> supplementati<strong>on</strong>encapsulati<strong>on</strong>, 103Israelvitamin D deficiency, 83Italyiodine fortificati<strong>on</strong>, 121ir<strong>on</strong> fortificati<strong>on</strong>, 108Jamaicafluoride fortificati<strong>on</strong>, 134Japanir<strong>on</strong> fortificati<strong>on</strong>, 108prevalence of vitamin B 12 deficiency,66selenium deficiency, 88thiamine deficiency, 70Kaschin-Beck Disease, 88–9Kenyaprevalence of vitamin B 12 deficiency,66vitamin B 12 supplementati<strong>on</strong>, 67Keshan Disease, 88Koreaselenium deficiency, 88Lacto-ovo vegetariansdefined, 63folate intake, 63risk of vitamin B 12 deficiency, 66Latin Americaprevalence of vitamin A deficiency,49zinc fortificati<strong>on</strong>, 125Legal Minimum Level (LmL). See Foodfortificati<strong>on</strong> levelsLower Reference Nutrient Intake,146Lower Threshold Intake (LTI), 146Maize flourir<strong>on</strong> fortificati<strong>on</strong>, 106Pan American Health Organizati<strong>on</strong>recommendati<strong>on</strong>, 107Malariaanaemia, 44effects of, 204336
INDEXMalaysiaiodine fortificati<strong>on</strong>, 121Maliiodine fortificati<strong>on</strong>, 121Mandatory fortificati<strong>on</strong>defined, 31Market-driven fortificati<strong>on</strong>, 171defined, 26, 28maximum micr<strong>on</strong>utrient c<strong>on</strong>tent,173safe maximum limit, 173Mass fortificati<strong>on</strong>, 294defined, 26–27Mass fortificati<strong>on</strong> programmesc<strong>on</strong>straints, 166enforcement, 168Feasible Fortificati<strong>on</strong> Levels,168fortificati<strong>on</strong> costs, 166Maximum Fortificati<strong>on</strong> Level (MFL).See Food fortificati<strong>on</strong> levelsMaximum Tolerable Level (MTL). SeeFood fortificati<strong>on</strong> levelsMeasles, 51Mexicofolic acid fortificati<strong>on</strong>, 128targeted fortificati<strong>on</strong>, 28Micr<strong>on</strong>utrient malnutriti<strong>on</strong>at-risk ages, 3comm<strong>on</strong> forms, 3defined, 3<strong>food</strong>-based strategies. See <strong>food</strong>-basedstrategiesMicr<strong>on</strong>utrient-poor processed <strong>food</strong>s, 5Micr<strong>on</strong>utrient-rich <strong>food</strong>sexamples, 4Milkiodine fortificati<strong>on</strong>, 122vitamin D fortificati<strong>on</strong>, 20, 130zinc fortificati<strong>on</strong>, 125Millennium Development Goals, xixMinimum Fortificati<strong>on</strong> Level (mFL).See Food fortificati<strong>on</strong> levelsM<strong>on</strong>itoringHousehold Minimum, 182household/individual, 179Maximum Tolerable Level (t),185Producti<strong>on</strong> Minimum, 182Quality Audit for Evaluati<strong>on</strong> ofC<strong>on</strong>formity (t), 184regulatory, 179Retail or Legal Minimum, 182M<strong>on</strong>itoring, commercial, 180,182label claims, 190minimum durability, 190M<strong>on</strong>itoring, external, 180, 188corroborating tests, 190inspecti<strong>on</strong>, 189Legal Minimum, 190Maximum Tolerable Level,189–90quantitative assay, 189technical auditing, 189M<strong>on</strong>itoring, household, 19130-cluster surveys, 192cross-secti<strong>on</strong>al surveys, 193lot quality assurance sampling,192market surveys, 193 (t), 195school surveys or censuses,193 (t)sentinel sites m<strong>on</strong>itoring, 192M<strong>on</strong>itoring, internal, 180good manufacturing practice (GMP),184quality assurance, 186quality c<strong>on</strong>trol, 186–87semi-quantitative assays, 188M<strong>on</strong>itoring, sampling, 187demanding intensity, 188normal intensity, 188relaxed intensity, 187Multiple fortificati<strong>on</strong>efficacy trials, 16Multiple micr<strong>on</strong>utrient deficienciesprevalence and risk factors, 91NaFeEDTA. See sodium ir<strong>on</strong> EDTANepalriboflavin deficiency, 73thiamine deficiency, 70New Zealandmandatory fortificati<strong>on</strong>, 32Niacinbioavailability of, 74defined, 73337
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSNiacin deficiencyhealth c<strong>on</strong>sequences of, 76pellagra, 74prevalence of, 74risk factors for, 74Niacin fortificati<strong>on</strong><strong>food</strong> vehicles, 128safety issues, 129Niacinamide, 129ULs, 129Nicaraguafolic acid fortificati<strong>on</strong>, 128vitamin A fortificati<strong>on</strong>, 116Nicotinic acid, 129ULs, 129Night blindness, 49Nutrient Reference Values (NRVs)defined, 172Nutriti<strong>on</strong>al equivalence, 26Osteomalaciavitamin D deficiency, 81Osteoporosis, 84Panamafolic acid fortificati<strong>on</strong>, 128Pellagra. See Niacin deficiencyPerutargeted fortificati<strong>on</strong>, 28Philippines Act Promoting SaltIodizati<strong>on</strong> Nati<strong>on</strong>wide. See <strong>food</strong>lawPhytic acidir<strong>on</strong> absorpti<strong>on</strong>, 102Potassium iodate, 118Potassium iodide, 118Promoti<strong>on</strong>advocacy, 225nutriti<strong>on</strong> educati<strong>on</strong>, 225social marketing, 225Protein–Energy Malnutriti<strong>on</strong> (PEM),5Recommended Dietary Allowance(RDA), 146Recommended Nutrient Intake (RNI)defined, 144Restorati<strong>on</strong>, 26Retinol, 49Riboflavinbioavailability of, 73defined, 71Riboflavin deficiencyhealth c<strong>on</strong>sequences of, 73prevalence of, 71risk factors for, 73Riboflavin fortificati<strong>on</strong><strong>food</strong> vehicles, 128safety issues, 129Riceir<strong>on</strong> fortificati<strong>on</strong>, 108vitamin A fortificati<strong>on</strong>, 116Rice fortificati<strong>on</strong> (ir<strong>on</strong>)difficulties of, 108Ricketsdefined, 81vitamin D deficiency, 81Safe Intake, 146Saltfluoridati<strong>on</strong>, 134iodine fortificati<strong>on</strong>, 119iodized, c<strong>on</strong>sumpti<strong>on</strong> rate,120iodized, stability of, 120<strong>with</strong> ir<strong>on</strong> and iodine. See dualfortificati<strong>on</strong>Salt iodizati<strong>on</strong>, 54, 119history of, 14processing, 119–20recommended levels, 159WHO programmes, 121Salt refining, 119Scandinaviaselenium deficiency, 88Scientific Committee for Foodof the European Community,147Scotlandfluoride fortificati<strong>on</strong>, 134Scurvy. See vitamin C deficiencySeleniumbioavailability of, 88defined, 86Selenium deficiencydefined, 88health c<strong>on</strong>sequences of, 88Kaschin-Beck Disease, 89338
INDEXKeshan Disease, 88prevalence of, 88risk factors for, 88Selenium fortificants, 133Selenium fortificati<strong>on</strong>, 133Seychelles, Thethiamine deficiency, 70Sicilyiodine fortificati<strong>on</strong>, 121Social marketingcommunicati<strong>on</strong>, 230defined, 229place, 230price, 230product positi<strong>on</strong>ing, 230promoti<strong>on</strong>, 230Sodium EDTAuses in ir<strong>on</strong> fortificati<strong>on</strong>, 101Sodium ir<strong>on</strong> EDTA (NaFeEDTA),102Sodium selenateselenium fortificants, 174South Africair<strong>on</strong> fortificati<strong>on</strong>, 16multiple fortificati<strong>on</strong>, 16targeted fortificati<strong>on</strong>, 28South-East Asiainadequate iodine nutriti<strong>on</strong>, 54prevalence of vitamin A deficiency,49Soy sauce & fish sauceir<strong>on</strong> fortificati<strong>on</strong>, 109SPS Agreement, 240, 322Sudaniodine fortificati<strong>on</strong>, 121Sugariodine fortificati<strong>on</strong>, 122vitamin A fortificati<strong>on</strong>, 116Supplementati<strong>on</strong>defined, 13Switzerlandsalt iodizati<strong>on</strong>, 14voluntary fortificati<strong>on</strong>, 35Tanzaniamultiple fortificati<strong>on</strong>, 17Targeted fortificati<strong>on</strong>, see <strong>food</strong>fortificati<strong>on</strong> levels, 169defined, 27TBT Agreement, 240, 322c<strong>on</strong>formity assessment procedure,324n<strong>on</strong>-discriminati<strong>on</strong>, 325standard, 324technical regulati<strong>on</strong>, 323Technological limitdefined, 163organoleptic properties, 163Thailandiodine fortificati<strong>on</strong>, 121ir<strong>on</strong> fortificati<strong>on</strong>, 105, 109prevalence of vitamin B 12 deficiency,66thiamine deficiency, 70Thiaminedefined, 68main sources of, 70Thiamine deficiencyberiberi, 68beriberi, dry, 71beriberi, wet, 71defined, 68prevalence of, 68risk factors, 70severe forms of, 71Wernicke–Korsakov syndrome,71Thiamine fortificati<strong>on</strong><strong>food</strong> vehicles, 128safety issues, 128Tolerable Upper Level (UL),146–47Transferrinreceptors, 46saturati<strong>on</strong>, 46Turkeyvitamin D deficiency, 83Undernourished mothersfortificati<strong>on</strong> for, 169United Kingdomfluoride fortificati<strong>on</strong>, 134prevalence of vitamin B 12 deficiency,66selenium fortificati<strong>on</strong>, 133voluntary fortificati<strong>on</strong>, 34United States Food and Nutriti<strong>on</strong>Board, 129339
GUIDELINES ON FOOD FORTIFICATION WITH MICRONUTRIENTSUnited States of Americaberiberi, 20calcium deficiency, 85dental caries, 89folic acid fortificati<strong>on</strong>, 14, 19, 128folic acid supplementati<strong>on</strong> trials,63iodine fortificati<strong>on</strong>, 17ir<strong>on</strong> fortificati<strong>on</strong>, 17ir<strong>on</strong> supplementati<strong>on</strong>, 48mandatory fortificati<strong>on</strong>, 32mass fortificati<strong>on</strong>, 27pellagra, 74prevalance of vitamin B 12 deficiency,66rickets, 83salt iodizati<strong>on</strong>, 14selenium fortificati<strong>on</strong>, 133vitamin C deficiency, 79vitamin C fortificati<strong>on</strong>, 130vitamin D deficiency, 83vitamin D fortificati<strong>on</strong>, 20voluntary fortificati<strong>on</strong>, 33zinc fortificati<strong>on</strong>, 125Vegetarianslacto-ovo vegetarians. See lacto-ovovegetariansrisk of vitamin B 12 deficiency, 66Venezuelair<strong>on</strong> fortificati<strong>on</strong>, 17, 99, 106–107prevalence of vitamin B 12 deficiency,66Viet Namir<strong>on</strong> fortificati<strong>on</strong>, 15, 109Vitamin Abeta carotene, 112retinol. See retinolsources of, 51Tolerable Upper Intake Levels(ULs), 117Vitamin A deficiencychild mortality, 51DALYs, 3defined, 49diarrhoea, 51health c<strong>on</strong>sequences of, 51maternal mortality, 52measles, 51mortality data, 3night blindness, 52pregnant women, 52prevalence of, 49risk factors for, 49Vitamin A fortificantsdefined, 111forms of, 112retinyl acetate, 111retinyl palmitates, 111Vitamin A fortificati<strong>on</strong>cereals and flours, 115efficacy trials, 16, 19margarines and oils, 113Vitamin A supplementati<strong>on</strong>safety issues, 117Vitamin B fortificants, 126folic acid, 126Vitamin B fortificati<strong>on</strong><strong>food</strong> vehicles, 128Vitamin B 6defined, 76Vitamin B 6 deficiencyhealth c<strong>on</strong>sequences of, 78prevalence of, 76risk factors for, 78Vitamin B 9 . See folateVitamin B 12defined, 64Vitamin B 12 deficiencydefined, 66health c<strong>on</strong>sequences of, 67helicobacter pylori infecti<strong>on</strong>, 66risk factors for, 66Vitamin Cbioavailability of, 80defined, 78Vitamin C deficiencyclinical symptoms of, 81prevalence of, 78risk factors for, 80Vitamin C fortificants, 130Vitamin C fortificati<strong>on</strong>special <strong>food</strong>s, 130Vitamin Dbioavailability of, 83defined, 81Vitamin D deficiencydefined, 81340
INDEXhealth c<strong>on</strong>sequences of, 84osteomalacia, 81prevalence of, 82rickets, 81risk factors for, 83Vitamin D fortificants, 130Vitamin D fortificati<strong>on</strong>efficacy trials, 20milk, 131rickets, 20special <strong>food</strong>s, 130Voluntary fortificati<strong>on</strong>, 33, 250risks, 34Wateriodine fortificati<strong>on</strong>, 121methods of iodine fortificati<strong>on</strong>, 121Western Pacificinadequate iodine nutriti<strong>on</strong>, 54prevalence of vitamin A deficiency,49Wheat flourcalcium fortificati<strong>on</strong>, 131ir<strong>on</strong> fortificati<strong>on</strong>, 105vitamin A fortificati<strong>on</strong>, 19, 115World Declarati<strong>on</strong> <strong>on</strong> Nutriti<strong>on</strong>, xviiiWorld Food Dietary AssessmentSystem, 153World Food Programme (WFP),fortificati<strong>on</strong> guidelines, 28World Health Assembly, 11World Health Organizati<strong>on</strong> (WHO), xvibreast milk intakes, 169CHOICE project, 208iodine deficiency indicators, 54salt iodizati<strong>on</strong>, 120Vitamin and Mineral Nutriti<strong>on</strong>Informati<strong>on</strong> System, 5World Health Report, xivWorld Summit for Children, 11Zambiavitamin A fortificati<strong>on</strong>, 14, 116Zimbabweprevalence of vitamin B 12 deficiency,66Zincbioavailability of, 59, 124defined, 57methods to increase absorpti<strong>on</strong>,125Zinc deficiencyassociati<strong>on</strong> <strong>with</strong> ir<strong>on</strong> deficiency, 58defined, 57dermatitis, 61diarrhoea, 61health c<strong>on</strong>sequences of, 61mental disturbances, 61prevalence of, 57risk factors for, 59Zinc fortificantsdefined, 124Zinc fortificati<strong>on</strong>special <strong>food</strong>s, 125341
Interest in micr<strong>on</strong>utrient malnutriti<strong>on</strong> has increased greatly over the last fewyears. One of the main reas<strong>on</strong>s is the realizati<strong>on</strong> that micr<strong>on</strong>utrient malnutriti<strong>on</strong>c<strong>on</strong>tributes substantially to the global burden of disease. Furthermore, althoughmicr<strong>on</strong>utrient malnutriti<strong>on</strong> is more frequent and severe in the developing worldand am<strong>on</strong>g disadvantaged populati<strong>on</strong>s, it also represents a public healthproblem in some industrialized countries. Measures to correct micr<strong>on</strong>utrientdeficiencies aim at ensuring c<strong>on</strong>sumpti<strong>on</strong> of a balanced diet that is adequate inevery nutrient. Unfortunately, this is far from being achieved everywhere sinceit requires universal access to adequate <strong>food</strong> and appropriate dietary habits.Food fortificati<strong>on</strong> has the dual advantage of being able to deliver nutrients tolarge segments of the populati<strong>on</strong> <strong>with</strong>out requiring radical changes in <strong>food</strong>c<strong>on</strong>sumpti<strong>on</strong> patterns.Drawing <strong>on</strong> several recent high quality publicati<strong>on</strong>s and programme experience<strong>on</strong> the subject, informati<strong>on</strong> <strong>on</strong> <strong>food</strong> fortificati<strong>on</strong> has been critically analysedand then translated into scientifically sound guidelines for applicati<strong>on</strong> in thefield. The main purpose of these guidelines is to assist countries in the designand implementati<strong>on</strong> of appropriate <strong>food</strong> fortificati<strong>on</strong> programmes. They areintended to be a resource for governments and agencies that are currentlyimplementing or c<strong>on</strong>sidering <strong>food</strong> fortificati<strong>on</strong>, and a source of informati<strong>on</strong> forscientists, technologists and the <strong>food</strong> industry. The guidelines are written froma nutriti<strong>on</strong> and public health perspective, to provide practical guidance <strong>on</strong> how<strong>food</strong> fortificati<strong>on</strong> should be implemented, m<strong>on</strong>itored and evaluated. They areprimarily intended for nutriti<strong>on</strong>-related public health programme managers, butshould also be useful to all those working to c<strong>on</strong>trol micr<strong>on</strong>utrient malnutriti<strong>on</strong>,including the <strong>food</strong> industry.The document is organized into four complementary secti<strong>on</strong>s. Part I introducesthe c<strong>on</strong>cept of <strong>food</strong> fortificati<strong>on</strong> as a potential strategy for the c<strong>on</strong>trol ofmicr<strong>on</strong>utrient malnutriti<strong>on</strong>. Part II summarizes the prevalence, causes, andc<strong>on</strong>sequences of micr<strong>on</strong>utrient deficiencies, and the public health benefitsof micr<strong>on</strong>utrient malnutriti<strong>on</strong> c<strong>on</strong>trol. It lays the groundwork for public healthpers<strong>on</strong>nel to assess the magnitude of the problem and the potential benefits offortificati<strong>on</strong> in their particular situati<strong>on</strong>. Part III provides technical informati<strong>on</strong><strong>on</strong> the various chemical forms of micr<strong>on</strong>utrients that can be used to fortify<strong>food</strong>s, and reviews prior experiences of their use in specific <strong>food</strong> vehicles. PartIV describes the key steps involved in designing, implementing, and sustainingfortificati<strong>on</strong> programmes. Starting <strong>with</strong> a determinati<strong>on</strong> of the amount ofnutrients to be added to <strong>food</strong>s, this process c<strong>on</strong>tinues <strong>with</strong> the implementati<strong>on</strong>of m<strong>on</strong>itoring and evaluating systems (including quality c<strong>on</strong>trol/qualityassurance procedures), followed by an estimati<strong>on</strong> of cost-effectiveness andcost–benefit ratios. The importance of, and strategies for, regulati<strong>on</strong> andinternati<strong>on</strong>al harm<strong>on</strong>izati<strong>on</strong>, communicati<strong>on</strong>, advocacy, c<strong>on</strong>sumer marketingand public educati<strong>on</strong> are also explained in some detail.ISBN 92 4 159401 2