Raman Spectroscopy of nanomaterials - institut de chimie et des ...
Raman Spectroscopy of nanomaterials - institut de chimie et des ...
Raman Spectroscopy of nanomaterials - institut de chimie et des ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
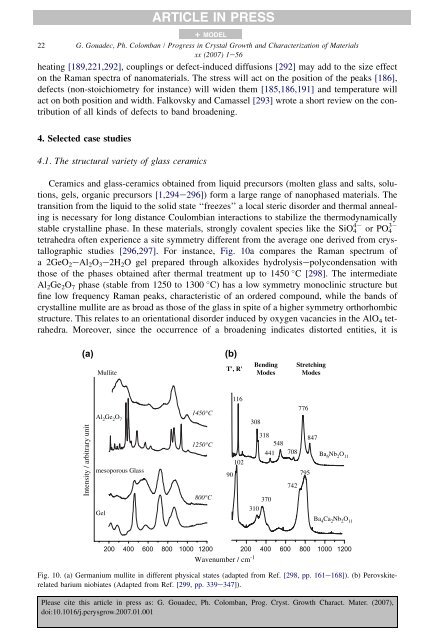
ARTICLE IN PRESS+ MODEL22 G. Goua<strong>de</strong>c, Ph. Colomban / Progress in Crystal Growth and Characterization <strong>of</strong> Materialsxx (2007) 1e56heating [189,221,292], couplings or <strong>de</strong>fect-induced diffusions [292] may add to the size effecton the <strong>Raman</strong> spectra <strong>of</strong> <strong>nanomaterials</strong>. The stress will act on the position <strong>of</strong> the peaks [186],<strong>de</strong>fects (non-stoichiom<strong>et</strong>ry for instance) will wi<strong>de</strong>n them [185,186,191] and temperature willact on both position and width. Falkovsky and Camassel [293] wrote a short review on the contribution<strong>of</strong> all kinds <strong>of</strong> <strong>de</strong>fects to band broa<strong>de</strong>ning.4. Selected case studies4.1. The structural vari<strong>et</strong>y <strong>of</strong> glass ceramicsCeramics and glass-ceramics obtained from liquid precursors (molten glass and salts, solutions,gels, organic precursors [1,294e296]) form a large range <strong>of</strong> nanophased materials. Th<strong>et</strong>ransition from the liquid to the solid state ‘‘freezes’’ a local steric disor<strong>de</strong>r and thermal annealingis necessary for long distance Coulombian interactions to stabilize the thermodynamically4 3stable crystalline phase. In these materials, strongly covalent species like the SiO 4 or PO 4t<strong>et</strong>rahedra <strong>of</strong>ten experience a site symm<strong>et</strong>ry different from the average one <strong>de</strong>rived from crystallographicstudies [296,297]. For instance, Fig. 10a compares the <strong>Raman</strong> spectrum <strong>of</strong>a 2GeO 2 eAl 2 O 3 e2H 2 O gel prepared through alkoxi<strong>de</strong>s hydrolysisepolycon<strong>de</strong>nsation withthose <strong>of</strong> the phases obtained after thermal treatment up to 1450 C [298]. The intermediateAl 2 Ge 2 O 7 phase (stable from 1250 to 1300 C) has a low symm<strong>et</strong>ry monoclinic structure butfine low frequency <strong>Raman</strong> peaks, characteristic <strong>of</strong> an or<strong>de</strong>red compound, while the bands <strong>of</strong>crystalline mullite are as broad as those <strong>of</strong> the glass in spite <strong>of</strong> a higher symm<strong>et</strong>ry orthorhombicstructure. This relates to an orientational disor<strong>de</strong>r induced by oxygen vacancies in the AlO 4 t<strong>et</strong>rahedra.Moreover, since the occurrence <strong>of</strong> a broa<strong>de</strong>ning indicates distorted entities, it is(a)Mullite(b)T', R'BendingMo<strong>de</strong>sStr<strong>et</strong>chingMo<strong>de</strong>sIntensity / arbitrary unitAl 2 Ge 2 O 7mesoporous GlassGel1450°C1250°C800°C11610290308318548441370310708776847Ba 6 Nb 2 O 11795742Ba 4 Ca 2 Nb 2 O 11200 400 600 800 10001200 200 400 600 800 1000 1200Wavenumber / cm -1Fig. 10. (a) Germanium mullite in different physical states (adapted from Ref. [298, pp. 161e168]). (b) Perovskiterelatedbarium niobiates (Adapted from Ref. [299, pp. 339e347]).Please cite this article in press as: G. Goua<strong>de</strong>c, Ph. Colomban, Prog. Cryst. Growth Charact. Mater. (2007),doi:10.1016/j.pcrysgrow.2007.01.001