Raman Spectroscopy of nanomaterials - institut de chimie et des ...
Raman Spectroscopy of nanomaterials - institut de chimie et des ...
Raman Spectroscopy of nanomaterials - institut de chimie et des ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
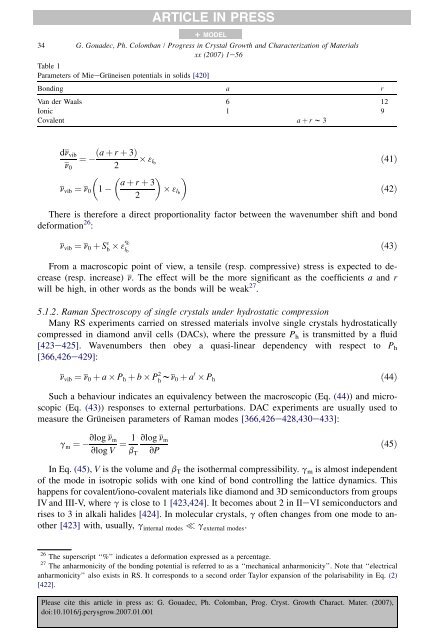
ARTICLE IN PRESS+ MODEL34 G. Goua<strong>de</strong>c, Ph. Colomban / Progress in Crystal Growth and Characterization <strong>of</strong> Materialsxx (2007) 1e56Table 1Param<strong>et</strong>ers <strong>of</strong> MieeGrüneisen potentials in solids [420]Bonding a rVan <strong>de</strong>r Waals 6 12Ionic 1 9Covalent a þ r w 3dn vib ða þ r þ 3Þ¼ 3 lbn 0 2 a þ r þ 3n vib ¼ n 01 3 lb2ð41Þð42ÞThere is therefore a direct proportionality factor b<strong>et</strong>ween the wavenumber shift and bond<strong>de</strong>formation 26 :n vib ¼ n 0 þ S 3 b 3% l bð43ÞFrom a macroscopic point <strong>of</strong> view, a tensile (resp. compressive) stress is expected to <strong>de</strong>crease(resp. increase) n. The effect will be the more significant as the coefficients a and rwill be high, in other words as the bonds will be weak 27 .5.1.2. <strong>Raman</strong> <strong>Spectroscopy</strong> <strong>of</strong> single crystals un<strong>de</strong>r hydrostatic compressionMany RS experiments carried on stressed materials involve single crystals hydrostaticallycompressed in diamond anvil cells (DACs), where the pressure P h is transmitted by a fluid[423e425]. Wavenumbers then obey a quasi-linear <strong>de</strong>pen<strong>de</strong>ncy with respect to P h[366,426e429]:n vib ¼ n 0 þ a P h þ b P 2 h wn 0 þ a 0 P hð44ÞSuch a behaviour indicates an equivalency b<strong>et</strong>ween the macroscopic (Eq. (44)) and microscopic(Eq. (43)) responses to external perturbations. DAC experiments are usually used tomeasure the Grüneisen param<strong>et</strong>ers <strong>of</strong> <strong>Raman</strong> mo<strong>de</strong>s [366,426e428,430e433]:g m ¼vlog n mvlog V ¼ 1 vlog n mb T vPð45ÞIn Eq. (45), V is the volume and b T the isothermal compressibility. g m is almost in<strong>de</strong>pen<strong>de</strong>nt<strong>of</strong> the mo<strong>de</strong> in isotropic solids with one kind <strong>of</strong> bond controlling the lattice dynamics. Thishappens for covalent/iono-covalent materials like diamond and 3D semiconductors from groupsIV and III-V, where g is close to 1 [423,424]. It becomes about 2 in IIeVI semiconductors andrises to 3 in alkali hali<strong>de</strong>s [424]. In molecular crystals, g <strong>of</strong>ten changes from one mo<strong>de</strong> to another[423] with, usually, g internal mo<strong>de</strong>s g external mo<strong>de</strong>s .26 The superscript ‘‘%’’ indicates a <strong>de</strong>formation expressed as a percentage.27 The anharmonicity <strong>of</strong> the bonding potential is referred to as a ‘‘mechanical anharmonicity’’. Note that ‘‘electricalanharmonicity’’ also exists in RS. It corresponds to a second or<strong>de</strong>r Taylor expansion <strong>of</strong> the polarisability in Eq. (2)[422].Please cite this article in press as: G. Goua<strong>de</strong>c, Ph. Colomban, Prog. Cryst. Growth Charact. Mater. (2007),doi:10.1016/j.pcrysgrow.2007.01.001