LHW Systems Review - Oxford Policy Management
LHW Systems Review - Oxford Policy Management
LHW Systems Review - Oxford Policy Management
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
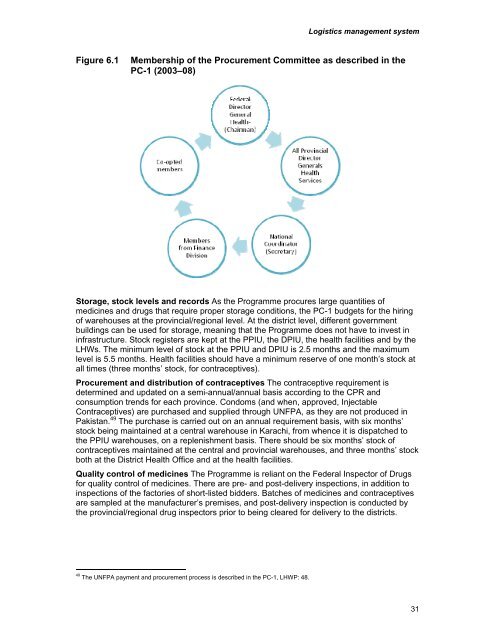
Logistics management systemFigure 6.1Membership of the Procurement Committee as described in thePC-1 (2003–08)Storage, stock levels and records As the Programme procures large quantities ofmedicines and drugs that require proper storage conditions, the PC-1 budgets for the hiringof warehouses at the provincial/regional level. At the district level, different governmentbuildings can be used for storage, meaning that the Programme does not have to invest ininfrastructure. Stock registers are kept at the PPIU, the DPIU, the health facilities and by the<strong>LHW</strong>s. The minimum level of stock at the PPIU and DPIU is 2.5 months and the maximumlevel is 5.5 months. Health facilities should have a minimum reserve of one month’s stock atall times (three months’ stock, for contraceptives).Procurement and distribution of contraceptives The contraceptive requirement isdetermined and updated on a semi-annual/annual basis according to the CPR andconsumption trends for each province. Condoms (and when, approved, InjectableContraceptives) are purchased and supplied through UNFPA, as they are not produced inPakistan. 49 The purchase is carried out on an annual requirement basis, with six months’stock being maintained at a central warehouse in Karachi, from whence it is dispatched tothe PPIU warehouses, on a replenishment basis. There should be six months’ stock ofcontraceptives maintained at the central and provincial warehouses, and three months’ stockboth at the District Health Office and at the health facilities.Quality control of medicines The Programme is reliant on the Federal Inspector of Drugsfor quality control of medicines. There are pre- and post-delivery inspections, in addition toinspections of the factories of short-listed bidders. Batches of medicines and contraceptivesare sampled at the manufacturer’s premises, and post-delivery inspection is conducted bythe provincial/regional drug inspectors prior to being cleared for delivery to the districts.49The UNFPA payment and procurement process is described in the PC-1, <strong>LHW</strong>P: 48.31