A Path to Prosperity New Directions for African Livestock
GALVmed Impetus Strategy Paper
GALVmed Impetus Strategy Paper
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
VICH<br />
The “International Cooperation on Harmonisation<br />
of Technical Requirements <strong>for</strong> Registration<br />
of Veterinary Medicinal Products,” or VICH, was<br />
launched in 1996 following the convening of an OIE<br />
ad hoc group <strong>to</strong> discuss harmonisation of veterinary<br />
medicinal products. Currently, VICH is a trilateral<br />
(EU–Japan–USA) programme with regula<strong>to</strong>ry<br />
authorities and industry experts from Australia,<br />
Canada and <strong>New</strong> Zealand participating as<br />
observers. These three regions and three observers<br />
represent 70% of the global market <strong>for</strong> animal<br />
health products. The OIE is an associate member of<br />
VICH.<br />
After 14 years of development the VICH is reaching<br />
out. VICH’s public meeting of June 2010 provided an<br />
opportunity <strong>to</strong> review progress <strong>to</strong> date and look <strong>to</strong><br />
the future. It was concluded that:<br />
> VICH guidelines were useful as they:<br />
• allow efficient use of resources by avoiding<br />
the need <strong>for</strong> creation of new technical<br />
standards;<br />
• <strong>for</strong>m an internationally recognised basis<br />
<strong>for</strong> mutual recognition;<br />
• avoid the risk of dual standards;<br />
• can be referenced by legisla<strong>to</strong>rs creating<br />
new regula<strong>to</strong>ry frameworks:<br />
> Capacity building <strong>for</strong> effective regulations<br />
<strong>for</strong> authorisation and control of veterinary<br />
medicines was essential be<strong>for</strong>e any VICH<br />
standards could be used and that OIE has a<br />
key role in capacity building through the<br />
Per<strong>for</strong>mance of Veterinary Services (PVS)<br />
Scheme;<br />
> There is concern in the developing world that<br />
VICH guidelines might not be achievable and fail<br />
<strong>to</strong> cover issues relevant <strong>to</strong> developing countries<br />
eg. diseases not found in VICH member<br />
countries or traditional medicines and that<br />
guidelines may be imposed on them without an<br />
adequate opportunity <strong>for</strong> engagement in their<br />
development;<br />
> There is need <strong>for</strong> raising awareness on VICH and<br />
the development of new guidelines relevant <strong>to</strong><br />
new stakeholders;<br />
> VICH should work with OIE <strong>to</strong> develop a<br />
framework <strong>for</strong> capacity building veterinary<br />
medicine regula<strong>to</strong>ry systems that fully<br />
integrates the standards developed by VICH;<br />
> RECs present a more effective target than<br />
individual countries.<br />
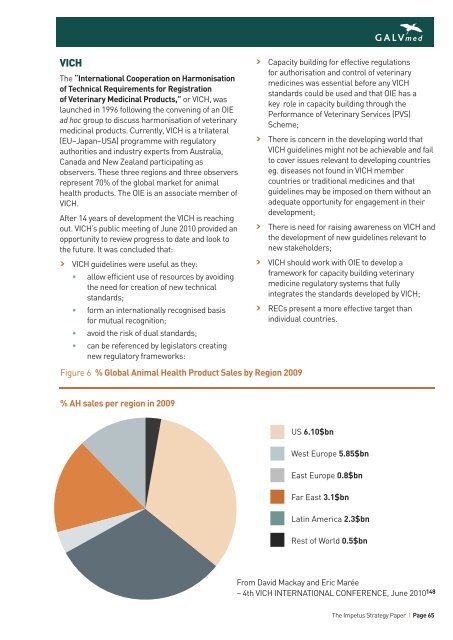
Figure 6 % Global Animal Health Product Sales by Region 2009<br />
% AH sales per region in 2009<br />
US 6.10$bn<br />
West Europe 5.85$bn<br />
East Europe 0.8$bn<br />
Far East 3.1$bn<br />
Latin America 2.3$bn<br />
Rest of World 0.5$bn<br />
From David Mackay and Eric Marée<br />
– 4th VICH INTERNATIONAL CONFERENCE, June 2010 148<br />
The Impetus Strategy Paper I Page 65