Tuyển tập 25 đề thi học sinh giỏi Hóa học lớp 9 (kèm đáp án) (by Dameva)
LINK BOX: https://app.box.com/s/o99ni841akj1du7thudv791t7d6gl511 LINK DOCS.GOOGLE: https://drive.google.com/file/d/1rxndliuwuCanNdIDkn77cCAwIFOWMvJF/view?usp=sharing
LINK BOX:
https://app.box.com/s/o99ni841akj1du7thudv791t7d6gl511
LINK DOCS.GOOGLE:
https://drive.google.com/file/d/1rxndliuwuCanNdIDkn77cCAwIFOWMvJF/view?usp=sharing
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
TUYỂN TẬP 50 ĐỀ THI HỌC SINH GIỎI MÔN HÓA HỌC LỚP 9 (<strong>kèm</strong> <strong>đáp</strong> <strong>án</strong>)<br />
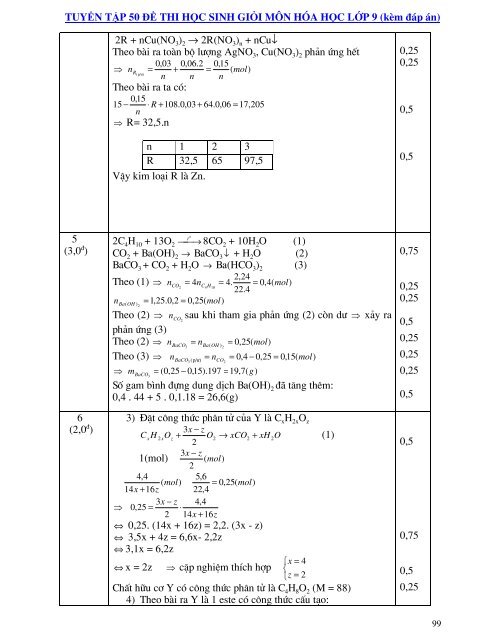
2R + nCu(NO 3 ) 2 → 2R(NO 3 ) n + nCu↓<br />
Theo bµi ra toµn bé l−îng AgNO 3 , Cu(NO 3 ) 2 phn øng hÕt<br />
⇒ n 0,03 0,06.2 0,15<br />
= +<br />
( mol)<br />
R (<br />
n n<br />
=<br />
p/−)<br />
n<br />
Theo bµi ra ta cã:<br />
0,15<br />
15 − ⋅ R + 108.0,03 + 64.0,06 = 17,205<br />
n<br />
⇒ R= 32,5.n<br />
n 1 2 3<br />
R 32,5 65 97,5<br />
VËy kim lo¹i R lµ Zn.<br />
0,<strong>25</strong><br />
0,<strong>25</strong><br />
0,5<br />
0,5<br />
5<br />
(3,0 ® )<br />
6<br />
(2,0 ® )<br />
2C 4 H 10 + 13O 2 ⎯⎯→<br />
t o<br />
8CO + 10H O (1)<br />
2 2<br />
CO 2 + Ba(OH) 2 → BaCO 3 ↓ + H 2 O (2)<br />
BaCO 3 + CO 2 + H 2 O → Ba(HCO 3 ) 2 (3)<br />
2,24<br />
Theo (1) ⇒ nCO = 4n<br />
4. 0,4( mol)<br />
2 C4H<br />
= =<br />
10<br />
22.4<br />
n Ba ( OH ) 2<br />
= 1,<strong>25</strong>.0,2 = 0,<strong>25</strong>( mol)<br />
Theo (2) ⇒ nCO<br />
sau khi tham gia phn øng (2) cßn d− ⇒ xy ra<br />
2<br />
phn øng (3)<br />
Theo (2) ⇒ nBaCO = n 0,<strong>25</strong>( )<br />
3 Ba( OH ) 2<br />
= mol<br />
Theo (3) ⇒ nBaCO n 0,4 0,<strong>25</strong> 0,15( mol)<br />
3 ( p/−)<br />
=<br />
CO<br />
= − =<br />
2<br />
⇒ m BaCO<br />
= (0,<strong>25</strong> − 0,15).197 = 19,7( g)<br />
3<br />
Sè gam b×nh ®ùng dung dÞch Ba(OH) 2 ®· t¨ng thªm:<br />
0,4 . 44 + 5 . 0,1.18 = 26,6(g)<br />
3) §Æt c«ng thøc ph©n tö cña Y lµ C x H 2x O z<br />
3x<br />
− z<br />
CxH<br />
2xOz<br />
+ O2<br />
→ xCO2<br />
+ xH<br />
2O<br />
(1)<br />
2<br />
3x − z<br />
1(mol) ( mol)<br />
2<br />
4,4<br />
5,6<br />
( mol)<br />
= 0,<strong>25</strong>( mol)<br />
14x + 16z<br />
22,4<br />
3x<br />
− z 4,4<br />
⇒ 0,<strong>25</strong> = ⋅<br />
2 14x<br />
+ 16z<br />
⇔ 0,<strong>25</strong>. (14x + 16z) = 2,2. (3x - z)<br />
⇔ 3,5x + 4z = 6,6x- 2,2z<br />
⇔ 3,1x = 6,2z<br />
⎧x<br />
= 4<br />
⇔ x = 2z ⇒ cÆp nghiÖm thÝch hîp ⎨<br />
⎩z<br />
= 2<br />
ChÊt h÷u c¬ Y cã c«ng thøc ph©n tö lµ C 4 H 8 O 2 (M = 88)<br />
4) Theo bµi ra Y lµ 1 este cã c«ng thøc cÊu t¹o:<br />
0,75<br />
0,<strong>25</strong><br />
0,<strong>25</strong><br />
0,5<br />
0,<strong>25</strong><br />
0,<strong>25</strong><br />
0,<strong>25</strong><br />
0,5<br />
0,5<br />
0,75<br />
0,5<br />
0,<strong>25</strong><br />
99