Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
the standard deviation from 16 blank determinations,<br />
m are the slopes <strong>of</strong> the analytical curves, MM is the<br />
molar mass <strong>of</strong> cipr<strong>of</strong>loxacin and V is the analyte volume<br />
deposited on the substrate (5 µL).<br />
The analytical curves showed linear dynamic ranges<br />
that extended from the ALOQ to at least 415 ng <strong>of</strong> cipr<strong>of</strong>loxacin.<br />
The analyte curve equations were Y = 0.41 ng -1<br />
X + 44 for substrates containing Th(NO 3 ) 4 and Y = 1.11<br />
ng -1 X + 59 for substrates containing CdCl 2 . The curves<br />
presented a homoscedastic behavior and their determination<br />
coefficients were close to the unity (R 2 >0.99).<br />
Evaluations <strong>of</strong> the repeatability were performed using<br />
two different masses <strong>of</strong> the analyte (83 and 331 ng). The<br />
relative standard deviation <strong>of</strong> the measured values varied<br />
from 3.2 to 6.6% what can be considered satisfactory<br />
for measurements from solid substrates.<br />
3.5. ap p l I c a t I o n <strong>of</strong> th e me t h o d<br />
The proposed SSRTP method was applied for the<br />
quantification <strong>of</strong> cipr<strong>of</strong>loxacin in one commercial pharmaceutical<br />
formulation. Other two simulated formulations<br />
were also analyzed. These simulated samples<br />
were prepared by mixing a known amount <strong>of</strong> cipr<strong>of</strong>loxacin<br />
standard with the pulverized pharmaceutical<br />
formulation <strong>of</strong> either gatifloxacin or moxifloxacin in<br />
Analyte<br />
www.brjac.com.br<br />
order to get cipr<strong>of</strong>loxacin/gatifloxacin or cipr<strong>of</strong>loxacin/<br />
moxifloxacin proportions <strong>of</strong> either 1/1 or 1/2 w/w. The<br />
recovery values were calculated based on the cipr<strong>of</strong>loxacin<br />
quantity indicated on the medicine instruction,<br />
which agreed with experimental results found using a<br />
reference HPLC with fluorescence detection 17 . Recovery<br />
tests were made using each one <strong>of</strong> the heavy atom<br />
salts as phosphorescence enhancer in order to get a<br />
comparison <strong>of</strong> performance. The tabulated values are<br />
averages <strong>of</strong> three different determinations performed<br />
in three different days and a Student t-test (at 95%<br />
confidence level) was used to statistically compare the<br />
experimental result with the reference one.<br />
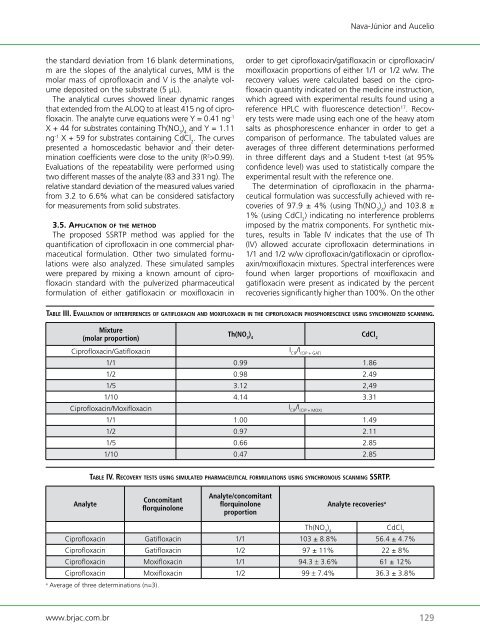
The determination <strong>of</strong> cipr<strong>of</strong>loxacin in the pharmaceutical<br />
formulation was successfully achieved with recoveries<br />
<strong>of</strong> 97.9 ± 4% (using Th(NO 3 ) 4 ) and 103.8 ±<br />
1% (using CdCl 2 ) indicating no interference problems<br />
imposed by the matrix components. For synthetic mixtures,<br />
results in Table IV indicates that the use <strong>of</strong> Th<br />
(IV) allowed accurate cipr<strong>of</strong>loxacin determinations in<br />
1/1 and 1/2 w/w cipr<strong>of</strong>loxacin/gatifloxacin or cipr<strong>of</strong>loxaxin/moxifloxacin<br />
mixtures. Spectral interferences were<br />
found when larger proportions <strong>of</strong> moxifloxacin and<br />
gatifloxacin were present as indicated by the percent<br />
recoveries significantly higher than 100%. On the other<br />
taBlE iii. Evaluation o f intErfErEnCEs o f g a t i f l o x aC i n a n d m o x i f l o x aC i n in thE C iP ro f l o x a C i n PhosPhorEsCEnCE u s i n g s y n C h ro n i zE d s C a n n i n g.<br />
Mixture<br />
(molar proportion)<br />
Cipr<strong>of</strong>loxacin/Gatifloxacin<br />
taBlE iv. rECovEry tEsts u s i n g simulatEd PharmaCEutiCal f o r m u l a t i o n s u s i n g s y n C h ro n o u s s C a n n i n g ssrtP.<br />
Concomitant<br />
florquinolone<br />
Analyte/concomitant<br />
florquinolone<br />
proportion<br />
Analyte recoveries a<br />
Th(NO ) 3 4<br />
CdCl2 Cipr<strong>of</strong>loxacin Gatifloxacin 1/1 103 ± 8.8% 56.4 ± 4.7%<br />
Cipr<strong>of</strong>loxacin Gatifloxacin 1/2 97 ± 11% 22 ± 8%<br />
Cipr<strong>of</strong>loxacin Moxifloxacin 1/1 94.3 ± 3.6% 61 ± 12%<br />
Cipr<strong>of</strong>loxacin Moxifloxacin 1/2 99 ± 7.4% 36.3 ± 3.8%<br />
a Average <strong>of</strong> three determinations (n=3).<br />
Th(NO 3 ) 4<br />
I CIP /I (CIP + GAT)<br />
CdCl 2<br />
1/1 0.99 1.86<br />
1/2 0.98 2.49<br />
1/5 3.12 2,49<br />
1/10 4.14 3.31<br />
Cipr<strong>of</strong>loxacin/Moxifloxacin<br />
I /I CIP (CIP + MOX)<br />
1/1 1.00 1.49<br />
1/2 0.97 2.11<br />
1/5 0.66 2.85<br />
1/10 0.47 2.85<br />
Nava-Júnior and Aucelio<br />
129