Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
taken to the laboratory and stored in a freezer until the<br />
analysis was performed. Samples containing pesticides<br />
above the MLR were analyzed by GC-MS to confirm the<br />
results. Furthermore, samples <strong>of</strong> potatoes in which pesticide<br />
residues were detected were peeled and the pulp<br />
and peel analyzed separately. A portion <strong>of</strong> the residue<br />
containing samples was also submitted to a cooking<br />
process and then peeled. The peel and pulp were then<br />
analyzed.<br />
All analyses were performed in triplicate.<br />
3. results a n d dIscussIon<br />
3.1. ch r o m a t o g r a p h Ic an a l y s Is<br />
Figure 1 represents the chromatogram <strong>of</strong> the GC-ECD<br />
analysis <strong>of</strong> a standard solution <strong>of</strong> the pesticides in acetonitrile<br />
at the established conditions. The peaks with<br />
t R equal to 4.1, 6.9, 8.1 and 9.5 minutes correspond<br />
to chlorpyrifos, λ-cyhalothrin, cypermethrin and deltamethrin,<br />
respectively. The second peak, with a retention<br />
time <strong>of</strong> 6.3 minutes corresponds to bifenthrin, the<br />
internal standard. The presence <strong>of</strong> a second peak for<br />
each <strong>of</strong> the pyrethroids is related to the conversion <strong>of</strong><br />
isomers during injection into the chromatograph [25].<br />
Therefore, the sum <strong>of</strong> the peak areas <strong>of</strong> both isomers<br />
was considered for quantification <strong>of</strong> each pyrethroid.<br />
fi g u rE 1. Ch ro m a t o g ra m o f a 500.0 µg l -1 standard s o l u t i o n o f thE<br />
aCtivE i n g rE d iE n t s in a C E t o n i t r i lE, w h E r E t r = 6.3 m i n: i n tE r n a l standard,<br />
t r = 4.1 m i n: ChlorPyrifos, t r = 6.9 m i n: λ- Cyhalothrin, t r = 8.1 m i n:<br />
CyPErmEthrin a n d t r = 9.5 m i n: dEltamEthrin.<br />
Quantification <strong>of</strong> pesticides in the extracts was performed<br />
by employing the internal standardization<br />
method. Calibration curves <strong>of</strong> each pesticide were constructed<br />
for the concentration interval <strong>of</strong> 6.0 to 750.0<br />
µg L -1 , obtaining correlation coefficients (r) greater than<br />
0.99.<br />
3.2. optImIzatIon o f t h e sle-ltp method<br />
In the optimization process <strong>of</strong> the SLE-LTP technique,<br />
www.brjac.com.br<br />
Dardengo et al.<br />
analyses were performed <strong>of</strong> the pesticides chlorpyrifos,<br />
λ-cyhalothrin, cypermethrin and deltamethrin in potatoes.<br />
Some factors which influenced the results <strong>of</strong> the<br />
technique, such as type <strong>of</strong> extraction mixture, time <strong>of</strong><br />
ultrasound, the ionic strength effect and fortification<br />
time were evaluated using fortified potato samples.<br />
The experimental planning <strong>of</strong> the mixtures was performed<br />
on the basis <strong>of</strong> volumetric proportions <strong>of</strong> water,<br />
acetonitrile and ethyl acetate, determined by the upper<br />
and lower limits <strong>of</strong> each solvent in the extraction<br />
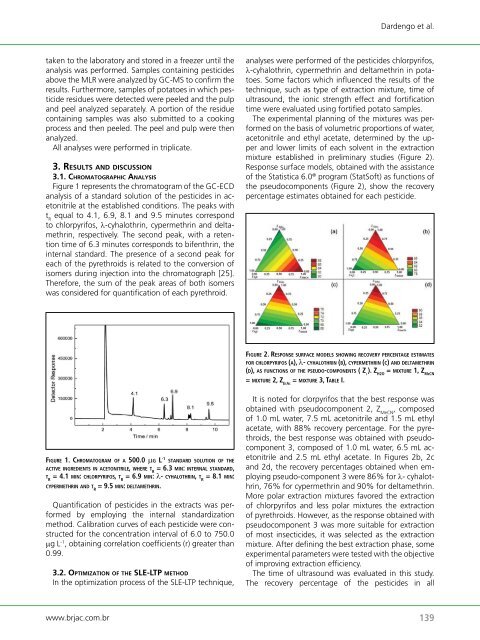
mixture established in preliminary studies (Figure 2).<br />
Response surface models, obtained with the assistance<br />
<strong>of</strong> the Statistica 6.0 ® program (StatS<strong>of</strong>t) as functions <strong>of</strong><br />
the pseudocomponents (Figure 2), show the recovery<br />
percentage estimates obtained for each pesticide.<br />
fi g u rE 2. rEsPonsE surfaCE m o d E l s s h o w i n g rECovEry P E rC E n t a g E EstimatEs<br />
f o r ChlorPyrifos (a), λ- Cyhalothrin (B), CyPErmEthrin (C) a n d dEltamEthrin<br />
(d), as f u n C t i o n s o f thE PsEudo-C o m P o n E n t s ( z i ). z h2o = m i x t u rE 1, z mECn<br />
= m i x t u rE 2, z EtaC = m i x t u rE 3, taBlE i.<br />
It is noted for clorpyrifos that the best response was<br />
obtained with pseudocomponent 2, Z MeCN , composed<br />
<strong>of</strong> 1.0 mL water, 7.5 mL acetonitrile and 1.5 mL ethyl<br />
acetate, with 88% recovery percentage. For the pyrethroids,<br />
the best response was obtained with pseudocomponent<br />
3, composed <strong>of</strong> 1.0 mL water, 6.5 mL acetonitrile<br />
and 2.5 mL ethyl acetate. In Figures 2b, 2c<br />
and 2d, the recovery percentages obtained when employing<br />
pseudo-component 3 were 86% for λ- cyhalothrin,<br />
76% for cypermethrin and 90% for deltamethrin.<br />
More polar extraction mixtures favored the extraction<br />
<strong>of</strong> chlorpyrifos and less polar mixtures the extraction<br />
<strong>of</strong> pyrethroids. However, as the response obtained with<br />
pseudocomponent 3 was more suitable for extraction<br />
<strong>of</strong> most insecticides, it was selected as the extraction<br />
mixture. After defining the best extraction phase, some<br />
experimental parameters were tested with the objective<br />
<strong>of</strong> improving extraction efficiency.<br />
The time <strong>of</strong> ultrasound was evaluated in this study.<br />
The recovery percentage <strong>of</strong> the pesticides in all<br />
139