Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
Brazilian Journal of Analytical Chemistry - BRJAC - Brazilian Journal ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Eberlin et al.<br />
<strong>of</strong> new and stable coatings has been produced [14]<br />
[15]. The advantages <strong>of</strong> these sol-gel coatings, such as<br />
better chemical and mechanical resistances (including<br />
thermal resistance), when compared with the commercial<br />
fibers, result from the chemical anchoring <strong>of</strong><br />
the sorbent phase onto the fused silica fiber. Briefly,<br />
sol-gel reactions are based on the hydrolysis and condensation<br />
<strong>of</strong> (generally) silicon alcoxides, leading to<br />
a three-dimensional glassy network. Organic compounds<br />
containing reactive functionalities (hydroxyl,<br />
for example) may take part <strong>of</strong> the reaction, giving rise<br />
to an organically modified silica (ormosil). The surface<br />
<strong>of</strong> the ormosil possesses hydroxyl groups, hence they<br />
are able to bind to the surface <strong>of</strong> the fused silica fiber,<br />
which has also superficial hydroxyl groups. The characteristics<br />
<strong>of</strong> an ormosil may be tailored according to<br />
the condensable organic compounds added. A sol-gel<br />
fiber based on aminopropyltrimethoxysilane (APTMS)<br />
and PDMS [16] has been described.<br />
Chromatographic analysis <strong>of</strong> CP <strong>of</strong>ten requires a derivatization<br />
step, since their acidic hydrogens give rise<br />
to peak tailing with poor detection limits and poor<br />
quantitation. The derivatizating agents usually employed<br />
are acetic anhydride [17] and reactive silicon<br />
compounds such as hexamethyldisilazane [18] and<br />
bis(trimethylsilyl)trifluoroacetamide [19], but their use<br />
introduces a new step into the analytical process.<br />
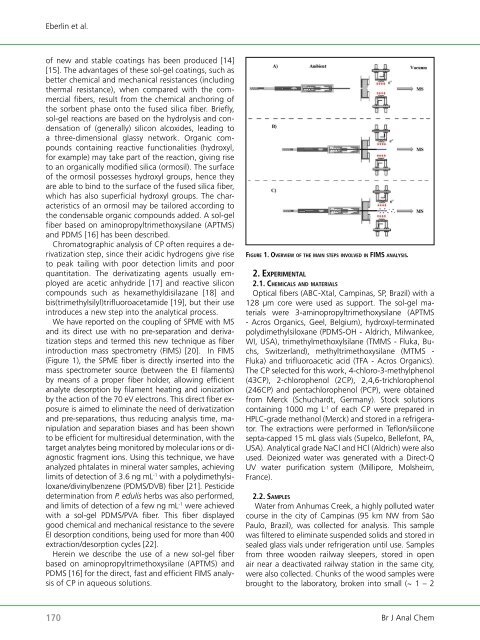
We have reported on the coupling <strong>of</strong> SPME with MS<br />
and its direct use with no pre-separation and derivatization<br />
steps and termed this new technique as fiber<br />
introduction mass spectrometry (FIMS) [20]. In FIMS<br />
(Figure 1), the SPME fiber is directly inserted into the<br />
mass spectrometer source (between the EI filaments)<br />
by means <strong>of</strong> a proper fiber holder, allowing efficient<br />
analyte desorption by filament heating and ionization<br />
by the action <strong>of</strong> the 70 eV electrons. This direct fiber exposure<br />
is aimed to eliminate the need <strong>of</strong> derivatization<br />
and pre-separations, thus reducing analysis time, manipulation<br />
and separation biases and has been shown<br />
to be efficient for multiresidual determination, with the<br />
target analytes being monitored by molecular ions or diagnostic<br />
fragment ions. Using this technique, we have<br />
analyzed phtalates in mineral water samples, achieving<br />
limits <strong>of</strong> detection <strong>of</strong> 3.6 ng mL -1 with a polydimethylsiloxane/divinylbenzene<br />
(PDMS/DVB) fiber [21]. Pesticide<br />
determination from P. edulis herbs was also performed,<br />
and limits <strong>of</strong> detection <strong>of</strong> a few ng mL -1 were achieved<br />
with a sol-gel PDMS/PVA fiber. This fiber displayed<br />
good chemical and mechanical resistance to the severe<br />
EI desorption conditions, being used for more than 400<br />
extraction/desorption cycles [22].<br />
Herein we describe the use <strong>of</strong> a new sol-gel fiber<br />
based on aminopropyltrimethoxysilane (APTMS) and<br />
PDMS [16] for the direct, fast and efficient FIMS analysis<br />
<strong>of</strong> CP in aqueous solutions.<br />
fi g u rE 1. ovErviEw o f thE m a i n stEPs i n v o l vE d in fims analysis.<br />
2. ExPErimEntal<br />
2.1. Ch E m i C a l s a n d m a t E r i a l s<br />
Optical fibers (ABC-Xtal, Campinas, SP, Brazil) with a<br />
128 μm core were used as support. The sol-gel materials<br />
were 3-aminopropyltrimethoxysilane (APTMS<br />
- Acros Organics, Geel, Belgium), hydroxyl-terminated<br />
polydimethylsiloxane (PDMS-OH - Aldrich, Milwankee,<br />
WI, USA), trimethylmethoxylsilane (TMMS - Fluka, Buchs,<br />
Switzerland), methyltrimethoxysilane (MTMS -<br />
Fluka) and trifluoroacetic acid (TFA - Acros Organics).<br />
The CP selected for this work, 4-chloro-3-methylphenol<br />
(43CP), 2-chlorophenol (2CP), 2,4,6-trichlorophenol<br />
(246CP) and pentachlorophenol (PCP), were obtained<br />
from Merck (Schuchardt, Germany). Stock solutions<br />
containing 1000 mg L -1 <strong>of</strong> each CP were prepared in<br />
HPLC-grade methanol (Merck) and stored in a refrigerator.<br />
The extractions were performed in Teflon/silicone<br />
septa-capped 15 mL glass vials (Supelco, Bellefont, PA,<br />
USA). <strong>Analytical</strong> grade NaCl and HCl (Aldrich) were also<br />
used. Deionized water was generated with a Direct-Q<br />
UV water purification system (Millipore, Molsheim,<br />
France).<br />
2.2. sa m P l E s<br />
Water from Anhumas Creek, a highly polluted water<br />
course in the city <strong>of</strong> Campinas (95 km NW from São<br />
Paulo, Brazil), was collected for analysis. This sample<br />
was filtered to eliminate suspended solids and stored in<br />
sealed glass vials under refrigeration until use. Samples<br />
from three wooden railway sleepers, stored in open<br />
air near a deactivated railway station in the same city,<br />
were also collected. Chunks <strong>of</strong> the wood samples were<br />
brought to the laboratory, broken into small (~ 1 – 2<br />
170 Br J Anal Chem