Prearranged Glycosides, Part 12, Intramolecular Mannosylations of ...
Prearranged Glycosides, Part 12, Intramolecular Mannosylations of ...
Prearranged Glycosides, Part 12, Intramolecular Mannosylations of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Helvetica Chimica Acta ± Vol. 83 2000) 2659<br />
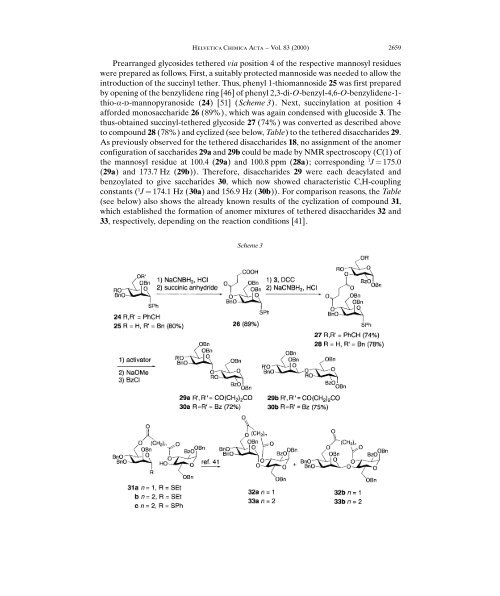
<strong>Prearranged</strong> glycosides tethered via position 4 <strong>of</strong> the respective mannosyl residues<br />
were prepared as follows. First, a suitably protected mannoside was needed to allow the<br />
introduction <strong>of</strong> the succinyl tether. Thus, phenyl 1-thiomannoside 25 was first prepared<br />
by opening <strong>of</strong> the benzylidene ring [46] <strong>of</strong> phenyl 2,3-di-O-benzyl-4,6-O-benzylidene-1thio-a-d-mannopyranoside<br />
24) [51] Scheme 3). Next, succinylation at position 4<br />
afforded monosaccharide 26 89%), which was again condensed with glucoside 3. The<br />
thus-obtained succinyl-tethered glycoside 27 74%) was converted as described above<br />
to compound 28 78%) and cyclized see below, Table) to the tethered disaccharides 29.<br />
As previously observed for the tethered disaccharides 18, no assignment <strong>of</strong> the anomer<br />
configuration <strong>of</strong> saccharides 29a and 29b could be made by NMR spectroscopy C1) <strong>of</strong><br />
the mannosyl residue at 100.4 29a) and 100.8 ppm 28a); corresponding 1 J ˆ 175.0<br />
29a) and 173.7 Hz 29b)). Therefore, disaccharides 29 were each deacylated and<br />
benzoylated to give saccharides 30, which now showed characteristic C,H-coupling<br />
constants 1 J ˆ 174.1 Hz 30a) and 156.9 Hz 30b)). For comparison reasons, the Table<br />
see below) also shows the already known results <strong>of</strong> the cyclization <strong>of</strong> compound 31,<br />
which established the formation <strong>of</strong> anomer mixtures <strong>of</strong> tethered disaccharides 32 and<br />
33, respectively, depending on the reaction conditions [41].<br />
Scheme 3