Prearranged Glycosides, Part 12, Intramolecular Mannosylations of ...
Prearranged Glycosides, Part 12, Intramolecular Mannosylations of ...
Prearranged Glycosides, Part 12, Intramolecular Mannosylations of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
counterpart and their comparison with those <strong>of</strong> galactosyl-derived analogues. For<br />
example, for disaccharides obtained from 1-thiogalactosides tethered via position 2 by<br />
succinate to position 3 <strong>of</strong> a glucose acceptor, the corresponding a-d-1 ! 4)-linked<br />
saccharides are ca. 7 ± <strong>12</strong> kcal/mol more stable than the respective b-d-1 ! 4)-linked<br />
ones [39]. Contrarily, compound 6 is calculated to be ca. 36 kcal/mol more stable than<br />
the corresponding b-d-linked disaccharide. Thus, solely 6 was formed in the cyclizations<br />
<strong>of</strong> prearranged saccharides 5.<br />
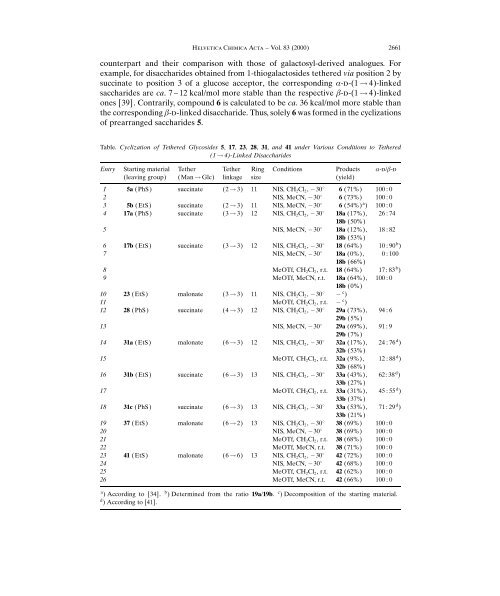
Table. Cyclization <strong>of</strong> Tethered <strong>Glycosides</strong> 5, 17, 23, 28, 31, and 41 under Various Conditions to Tethered<br />
1! 4)-Linked Disaccharides<br />
Entry Starting material<br />
leaving group)<br />
Tether<br />
Man ! Glc)<br />
Tether<br />
linkage<br />
Ring<br />
size<br />
Conditions Products<br />
yield)<br />
a-d/b-d<br />
1 5a PhS) succinate 2 ! 3) 11 NIS, CH2Cl2, 308 6 71%) 100 : 0<br />
2 NIS, MeCN, 308 6 73%) 100 : 0<br />
3 5b EtS) succinate 2 ! 3) 11 NIS, MeCN, 308 6 54%) a ) 100 : 0<br />
4 17a PhS) succinate 3 ! 3) <strong>12</strong> NIS, CH2Cl2, 308 18a 17%),<br />
18b 50%)<br />
26 : 74<br />
5 NIS, MeCN, 308 18a <strong>12</strong>%),<br />
18b 53%)<br />
18 : 82<br />
6 17b EtS) succinate 3 ! 3) <strong>12</strong> NIS, CH2Cl2, 308 18 64%) 10 : 90b )<br />
7 NIS, MeCN, 308 18a 0%),<br />
18b 66%)<br />
0 : 100<br />
8 MeOTf, CH2Cl2, r.t. 18 64%) 17 : 83b )<br />
9 MeOTf, MeCN, r.t. 18a 64%),<br />
18b 0%)<br />
100 : 0<br />
10 23 EtS) malonate 3 ! 3) 11 NIS, CH2Cl2, 308 c )<br />
11 MeOTf, CH2Cl2, r.t. c )<br />
<strong>12</strong> 28 PhS) succinate 4 ! 3) <strong>12</strong> NIS, CH2Cl2, 308 29a 73%),<br />
29b 5%)<br />
94 : 6<br />
13 NIS, MeCN, 308 29a 69%),<br />
29b 7%)<br />
91 : 9<br />
14 31a EtS) malonate 6 ! 3) <strong>12</strong> NIS, CH2Cl2, 308 32a 17%),<br />
32b 53%)<br />
24 : 76d )<br />
15 MeOTf, CH2Cl2, r.t. 32a 9%),<br />
32b 68%)<br />
<strong>12</strong> : 88d )<br />
16 31b EtS) succinate 6 ! 3) 13 NIS, CH2Cl2, 308 33a 43%),<br />
33b 27%)<br />
62: 38d )<br />
17 MeOTf, CH2Cl2, r.t. 33a 31%),<br />
33b 37%)<br />
45: 55 d )<br />
18 31c PhS) succinate 6 ! 3) 13 NIS, CH2Cl2, 308 33a 53%),<br />
33b 21%)<br />
71 : 29d )<br />
19 37 EtS) malonate 6 ! 2) 13 NIS, CH2Cl2, 308 38 69%) 100 : 0<br />
20 NIS, MeCN, 308 38 69%) 100 : 0<br />
21 MeOTf, CH2Cl2, r.t. 38 68%) 100 : 0<br />
22 MeOTf, MeCN, r.t. 38 71%) 100 : 0<br />
23 41 EtS) malonate 6 ! 6) 13 NIS, CH2Cl2, 308 42 72%) 100 : 0<br />
24 NIS, MeCN, 308 42 68%) 100 : 0<br />
25 MeOTf, CH2Cl2, r.t. 42 62%) 100 : 0<br />
26 MeOTf, MeCN, r.t. 42 66%) 100 : 0<br />
a ) According to [34]. b ) Determined from the ratio 19a/19b. c ) Decomposition <strong>of</strong> the starting material.<br />
d ) According to [41].<br />
Helvetica Chimica Acta ± Vol. 83 2000) 2661