Full Journal - Journal of Cell and Molecular Biology - Haliç Üniversitesi
Full Journal - Journal of Cell and Molecular Biology - Haliç Üniversitesi
Full Journal - Journal of Cell and Molecular Biology - Haliç Üniversitesi
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
1991, Russel <strong>and</strong> Synder, 1968; Pegg <strong>and</strong> Williams,<br />
1968). Ornithine decarboxylase is a unique enzyme in<br />
many respects. First, it is one <strong>of</strong> the enzymes having<br />
extremely short life with a very rapid turnover rate<br />
(t1/2 is from 10 to 20 minutes) <strong>and</strong> it is present in very<br />
small amounts in normal growing cells. Its activity<br />
can be increased many folds within a few hours <strong>of</strong><br />
exposure to trophic stimuli (Janne et al., 1991). Such<br />
stimuli include hormones, various drugs, tissue<br />
regeneration <strong>and</strong> growth factors commonly found in<br />
serum. Even after such stimulation, ODC remains<br />
only a very small fraction <strong>of</strong> the total cellular protein<br />
ranging from 0.01% <strong>of</strong> the cytosolic protein in<br />
<strong>and</strong>rogen - stimulated mouse kidneys to 0.00012 % in<br />
thioacetamide stimulated rat liver. It turned out that<br />
by any definition this enzyme is a low abundance<br />
Polyamines 61<br />
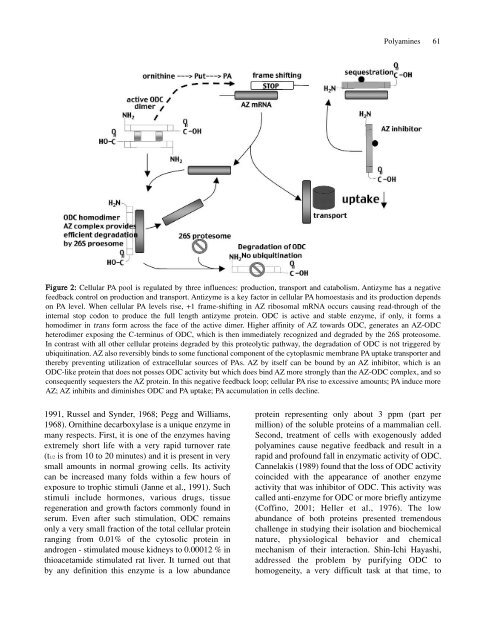
Figure 2: <strong>Cell</strong>ular PA pool is regulated by three influences: production, transport <strong>and</strong> catabolism. Antizyme has a negative<br />
feedback control on production <strong>and</strong> transport. Antizyme is a key factor in cellular PA homoestasis <strong>and</strong> its production depends<br />
on PA level. When cellular PA levels rise, +1 frame-shifting in AZ ribosomal mRNA occurs causing read-through <strong>of</strong> the<br />
internal stop codon to produce the full length antizyme protein. ODC is active <strong>and</strong> stable enzyme, if only, it forms a<br />
homodimer in trans form across the face <strong>of</strong> the active dimer. Higher affinity <strong>of</strong> AZ towards ODC, generates an AZ-ODC<br />
heterodimer exposing the C-terminus <strong>of</strong> ODC, which is then immediately recognized <strong>and</strong> degraded by the 26S proteosome.<br />
In contrast with all other cellular proteins degraded by this proteolytic pathway, the degradation <strong>of</strong> ODC is not triggered by<br />
ubiquitination. AZ also reversibly binds to some functional component <strong>of</strong> the cytoplasmic membrane PA uptake transporter <strong>and</strong><br />
thereby preventing utilization <strong>of</strong> extracellular sources <strong>of</strong> PAs. AZ by itself can be bound by an AZ inhibitor, which is an<br />
ODC-like protein that does not posses ODC activity but which does bind AZ more strongly than the AZ-ODC complex, <strong>and</strong> so<br />
consequently sequesters the AZ protein. In this negative feedback loop; cellular PA rise to excessive amounts; PA induce more<br />
AZ; AZ inhibits <strong>and</strong> diminishes ODC <strong>and</strong> PA uptake; PA accumulation in cells decline.<br />
protein representing only about 3 ppm (part per<br />
million) <strong>of</strong> the soluble proteins <strong>of</strong> a mammalian cell.<br />
Second, treatment <strong>of</strong> cells with exogenously added<br />
polyamines cause negative feedback <strong>and</strong> result in a<br />
rapid <strong>and</strong> pr<strong>of</strong>ound fall in enzymatic activity <strong>of</strong> ODC.<br />
Cannelakis (1989) found that the loss <strong>of</strong> ODC activity<br />
coincided with the appearance <strong>of</strong> another enzyme<br />
activity that was inhibitor <strong>of</strong> ODC. This activity was<br />
called anti-enzyme for ODC or more briefly antizyme<br />
(C<strong>of</strong>fino, 2001; Heller et al., 1976). The low<br />
abundance <strong>of</strong> both proteins presented tremendous<br />
challenge in studying their isolation <strong>and</strong> biochemical<br />
nature, physiological behavior <strong>and</strong> chemical<br />
mechanism <strong>of</strong> their interaction. Shin-Ichi Hayashi,<br />
addressed the problem by purifying ODC to<br />
homogeneity, a very difficult task at that time, to