Full Journal - Journal of Cell and Molecular Biology - Haliç Üniversitesi
Full Journal - Journal of Cell and Molecular Biology - Haliç Üniversitesi
Full Journal - Journal of Cell and Molecular Biology - Haliç Üniversitesi
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
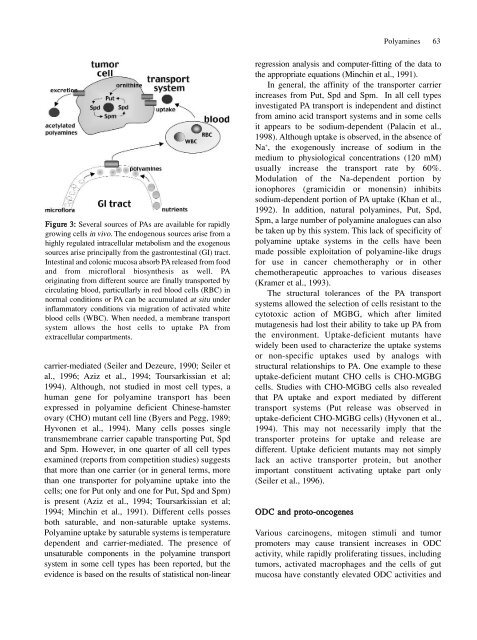
Figure 3: Several sources <strong>of</strong> PAs are available for rapidly<br />
growing cells in vivo. The endogenous sources arise from a<br />
highly regulated intracellular metabolism <strong>and</strong> the exogenous<br />
sources arise principally from the gastrontestinal (GI) tract.<br />
Intestinal <strong>and</strong> colonic mucosa absorb PA released from food<br />
<strong>and</strong> from micr<strong>of</strong>loral biosynthesis as well. PA<br />
originating from different source are finally transported by<br />
circulating blood, particullarly in red blood cells (RBC) in<br />
normal conditions or PA can be accumulated at situ under<br />
inflammatory conditions via migration <strong>of</strong> activated white<br />
blood cells (WBC). When needed, a membrane transport<br />
system allows the host cells to uptake PA from<br />
extracellular compartments.<br />
carrier-mediated (Seiler <strong>and</strong> Dezeure, 1990; Seiler et<br />
al., 1996; Aziz et al., 1994; Toursarkissian et al;<br />
1994). Although, not studied in most cell types, a<br />
human gene for polyamine transport has been<br />
expressed in polyamine deficient Chinese-hamster<br />
ovary (CHO) mutant cell line (Byers <strong>and</strong> Pegg, 1989;<br />
Hyvonen et al., 1994). Many cells posses single<br />
transmembrane carrier capable transporting Put, Spd<br />
<strong>and</strong> Spm. However, in one quarter <strong>of</strong> all cell types<br />
examined (reports from competition studies) suggests<br />
that more than one carrier (or in general terms, more<br />
than one transporter for polyamine uptake into the<br />
cells; one for Put only <strong>and</strong> one for Put, Spd <strong>and</strong> Spm)<br />
is present (Aziz et al., 1994; Toursarkissian et al;<br />
1994; Minchin et al., 1991). Different cells posses<br />
both saturable, <strong>and</strong> non-saturable uptake systems.<br />
Polyamine uptake by saturable systems is temperature<br />
dependent <strong>and</strong> carrier-mediated. The presence <strong>of</strong><br />
unsaturable components in the polyamine transport<br />
system in some cell types has been reported, but the<br />
evidence is based on the results <strong>of</strong> statistical non-linear<br />
regression analysis <strong>and</strong> computer-fitting <strong>of</strong> the data to<br />
the appropriate equations (Minchin et al., 1991).<br />
In general, the affinity <strong>of</strong> the transporter carrier<br />
increases from Put, Spd <strong>and</strong> Spm. In all cell types<br />
investigated PA transport is independent <strong>and</strong> distinct<br />
from amino acid transport systems <strong>and</strong> in some cells<br />
it appears to be sodium-dependent (Palacin et al.,<br />
1998). Although uptake is observed, in the absence <strong>of</strong><br />
Na + , the exogenously increase <strong>of</strong> sodium in the<br />
medium to physiological concentrations (120 mM)<br />
usually increase the transport rate by 60%.<br />
Modulation <strong>of</strong> the Na-dependent portion by<br />
ionophores (gramicidin or monensin) inhibits<br />
sodium-dependent portion <strong>of</strong> PA uptake (Khan et al.,<br />
1992). In addition, natural polyamines, Put, Spd,<br />
Spm, a large number <strong>of</strong> polyamine analogues can also<br />
be taken up by this system. This lack <strong>of</strong> specificity <strong>of</strong><br />
polyamine uptake systems in the cells have been<br />
made possible exploitation <strong>of</strong> polyamine-like drugs<br />
for use in cancer chemotheraphy or in other<br />
chemotherapeutic approaches to various diseases<br />
(Kramer et al., 1993).<br />
The structural tolerances <strong>of</strong> the PA transport<br />
systems allowed the selection <strong>of</strong> cells resistant to the<br />
cytotoxic action <strong>of</strong> MGBG, which after limited<br />
mutagenesis had lost their ability to take up PA from<br />
the environment. Uptake-deficient mutants have<br />
widely been used to characterize the uptake systems<br />
or non-specific uptakes used by analogs with<br />
structural relationships to PA. One example to these<br />
uptake-deficient mutant CHO cells is CHO-MGBG<br />
cells. Studies with CHO-MGBG cells also revealed<br />
that PA uptake <strong>and</strong> export mediated by different<br />
transport systems (Put release was observed in<br />
uptake-deficient CHO-MGBG cells) (Hyvonen et al.,<br />
1994). This may not necessarily imply that the<br />
transporter proteins for uptake <strong>and</strong> release are<br />
different. Uptake deficient mutants may not simply<br />
lack an active transporter protein, but another<br />
important constituent activating uptake part only<br />
(Seiler et al., 1996).<br />
ODC <strong>and</strong> proto-oncogenes<br />
Polyamines 63<br />
Various carcinogens, mitogen stimuli <strong>and</strong> tumor<br />
promoters may cause transient increases in ODC<br />
activity, while rapidly proliferating tissues, including<br />
tumors, activated macrophages <strong>and</strong> the cells <strong>of</strong> gut<br />
mucosa have constantly elevated ODC activities <strong>and</strong>