PUBLIC - Croatia, the War, and the Future
PUBLIC - Croatia, the War, and the Future
PUBLIC - Croatia, the War, and the Future
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
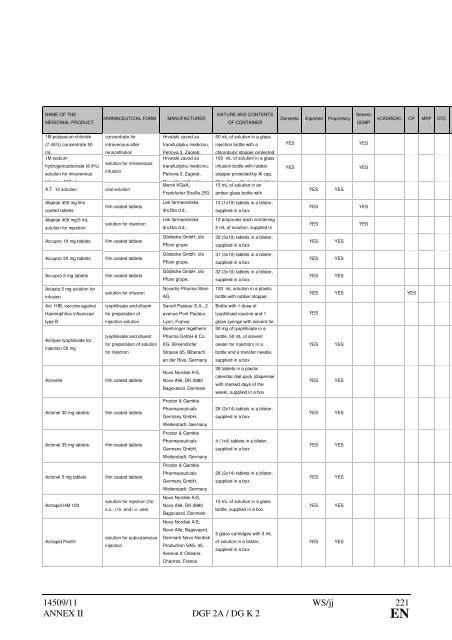
NAME OF THE<br />
MEDICINAL PRODUCT<br />
1M potassium-chloride<br />
(7.45%) concentrate 50<br />
mL<br />
1M sodium<br />
hydrogencarbonate (8.4%)<br />
solution for intravenous<br />
infusion, 100 mL<br />
PHARMACEUTICAL FORM MANUFACTURER<br />
concentrate for<br />
intravenous after<br />
reconstitution<br />
solution for intravenous<br />
infusion<br />
A.T. 10 solution oral solution<br />
Abaktal 400 mg film<br />
coated tablets<br />
Abaktal 400 mg/5 mL<br />
solution for injection<br />
film coated tablets<br />
solution for injection<br />
Accupro 10 mg tablets film coated tablets<br />
Accupro 20 mg tablets film coated tablets<br />
Accupro 5 mg tablets film coated tablets<br />
Aclasta 5 mg solution for<br />
infusion<br />
Act HIB, vaccine against<br />
Haemophilus influenzae<br />
type B<br />
Actilyse lyophilisate for<br />
injection 50 mg<br />
solution for infusion<br />
lyophilisate <strong>and</strong> diluent<br />
for preparation of<br />
injection solution<br />
lyophilisate <strong>and</strong> diluent<br />
for preparation of solution<br />
for injection<br />
Activelle film coated tablets<br />
Actonel 30 mg tablets film coated tablets<br />
Actonel 35 mg tablets film coated tablets<br />
Actonel 5 mg tablets film coated tablets<br />
Actrapid HM 100<br />
Actrapid Penfill<br />
solution for injection (for<br />
s.c., i.m. <strong>and</strong> i.v. use)<br />
solution for subcutaneous<br />
injection<br />
Hrvatski zavod za<br />
transfuzijsku medicinu,<br />
Petrova 3, Zagreb,<br />
Hrvatski zavod za<br />
transfuzijsku medicinu,<br />
Petrova 3, Zagreb,<br />
Republic of <strong>Croatia</strong><br />
Merck KGaA,<br />
Frankfurter Straße 250,<br />
Lek Darmstadt, farmacevtska Germany<br />
družba d.d.,<br />
Lek Verovškova farmacevtska 57,<br />
družba d.d.,<br />
Verovškova Gödecke GmbH, 57, dio<br />
Pfizer grupe,<br />
Karlsruhe, Gödecke GmbH, Germany dio<br />
Pfizer grupe,<br />
Karlsruhe, Gödecke GmbH, Germany dio<br />
Pfizer grupe,<br />
Novartis Karlsruhe, Pharma Germany Stein<br />
AG,<br />
Sanofi Schaffhauserstrasse,<br />
Pasteur S.A., 2<br />
avenue Pont Pasteur,<br />
Lyon, France<br />
Boehringer Ingelheim<br />
Pharma GmbH & Co.<br />
KG, Birkendorfer<br />
Strasse 65, Biberach<br />
an der Riss, Germany<br />
Novo Nordisk A/S,<br />
Novo Allé, DK-2880<br />
Bagsvaerd, Denmark<br />
Procter & Gamble<br />
Pharmaceuticals<br />
Germany GmbH,<br />
Weiterstadt, Germany<br />
Procter & Gamble<br />
Pharmaceuticals<br />
Germany GmbH,<br />
Weiterstadt, Germany<br />
Procter & Gamble<br />
Pharmaceuticals<br />
Germany GmbH,<br />
Weiterstadt, Germany<br />
Novo Nordisk A/S,<br />
Novo Allé, DK-2880<br />
Bagsvaerd, Denmark<br />
Novo Nordisk A/S,<br />
Novo Alle, Bagsvaerd,<br />
Denmark Novo Nordisk<br />
Production SAS, 45,<br />
Avenue d' Orleans,<br />
Chartres, France<br />
NATURE AND CONTENTS<br />
OF CONTAINER<br />
50 mL of solution in a glass<br />
injection bottle with a<br />
chlorobutyl stopper protected<br />
100 mL of solution in a glass<br />
infusion bottle with rubber<br />
stopper protected by Al cap,<br />
20 bottles with plastic holders<br />
15 mL of solution in an<br />
amber glass bottle with<br />
plastic<br />
10 (1x10)<br />
dropper<br />
tablets<br />
attachment,<br />
in a blister,<br />
supplied in a box<br />
10 ampoules each containing<br />
5 mL of solution, supplied in<br />
a<br />
30<br />
box<br />
(3x10) tablets in a blister,<br />
supplied in a box<br />
31 (3x10) tablets in a blister,<br />
supplied in a box<br />
32 (3x10) tablets in a blister,<br />
supplied in a box<br />
100 mL solution in a plastic<br />
bottle with rubber stopper,<br />
aluminum Bottle with ring 1 dose <strong>and</strong> of plastic<br />
lyophilised vaccine <strong>and</strong> 1<br />
glass syringe with solvent for<br />
50 mg of lyophilisate in a<br />
bottle, 50 mL of solvent<br />
(water for injection) in a<br />
bottle <strong>and</strong> a transfer needle,<br />
supplied in a box<br />
28 tablets in a plastic<br />
calendar dial pack (dispenser<br />
with marked days of <strong>the</strong><br />
week), supplied in a box<br />
28 (2x14) tablets in a blister,<br />
supplied in a box<br />
4 (1x4) tablets in a blister,<br />
supplied in a box<br />
28 (2x14) tablets in a blister,<br />
supplied in a box<br />
10 mL of solution in a glass<br />
bottle, supplied in a box<br />
5 glass cartridges with 3 mL<br />
of solution in a blister,<br />
supplied in a box<br />
Domestic Imported Proprietary Generic<br />
14509/11 WS/jj 221<br />
ANNEX II DGF 2A / DG K 2 EN<br />
UDMP<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
nCADREAC CP MRP OTC H<br />
YES YES YES<br />
YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES