PUBLIC - Croatia, the War, and the Future
PUBLIC - Croatia, the War, and the Future
PUBLIC - Croatia, the War, and the Future
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
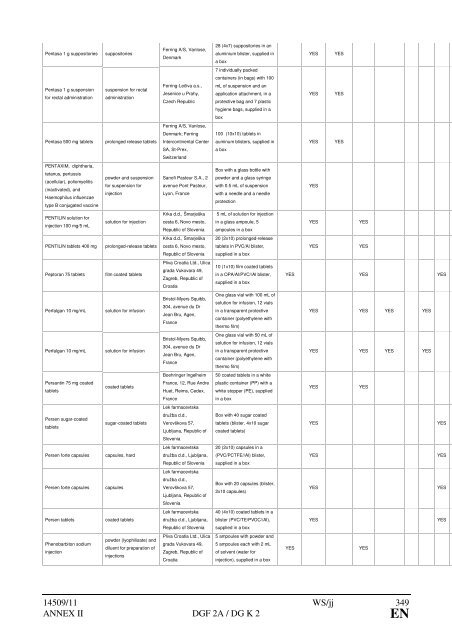
Pentasa 1 g suppositories suppositories<br />
Pentasa 1 g suspension<br />
for rectal administration<br />
suspension for rectal<br />
administration<br />
Pentasa 500 mg tablets prolonged release tablets<br />
PENTAXIM, diph<strong>the</strong>ria,<br />
tetanus, pertussis<br />
(acellular), poliomyelitis<br />
(inactivated), <strong>and</strong><br />
Haemophilus influenzae<br />
type B conjugated vaccine<br />
PENTILIN solution for<br />
injection 100 mg/5 mL<br />
powder <strong>and</strong> suspension<br />
for suspension for<br />
injection<br />
solution for injection<br />
PENTILIN tablets 400 mg prolonged-release tablets<br />
Peptoran 75 tablets film coated tablets<br />
Perfalgan 10 mg/mL solution for infusion<br />
Perfalgan 10 mg/mL solution for infusion<br />
Persantin 75 mg coated<br />
tablets<br />
Persen sugar-coated<br />
tablets<br />
coated tablets<br />
sugar-coated tablets<br />
Persen forte capsules capsules, hard<br />
Persen forte capsules capsules<br />
Persen tablets coated tablets<br />
Phenobarbiton sodium<br />
injection<br />
powder (lyophilisate) <strong>and</strong><br />
diluent for preparation of<br />
injections<br />
Ferring A/S, Vanlose,<br />
Denmark<br />
Ferring-Lečiva a.s.,<br />
Jesenice u Prahy,<br />
Czech Republic<br />
Ferring A/S, Vanlose,<br />
Denmark; Ferring<br />
Intercontinental Center<br />
SA, St-Prex,<br />
Switzerl<strong>and</strong><br />
Sanofi Pasteur S.A., 2<br />
avenue Pont Pasteur,<br />
Lyon, France<br />
Krka d.d., Šmarješka<br />
cesta 6, Novo mesto,<br />
Republic of Slovenia<br />
Krka d.d., Šmarješka<br />
cesta 6, Novo mesto,<br />
Republic of Slovenia<br />
Pliva <strong>Croatia</strong> Ltd., Ulica<br />
grada Vukovara 49,<br />
Zagreb, Republic of<br />
<strong>Croatia</strong><br />
Bristol-Myers Squibb,<br />
304, avenue du Dr<br />
Jean Bru, Agen,<br />
France<br />
Bristol-Myers Squibb,<br />
304, avenue du Dr<br />
Jean Bru, Agen,<br />
France<br />
Boehringer Ingelheim<br />
France, 12, Rue Andre<br />
Huet, Reims, Cedex,<br />
France<br />
Lek farmacevtska<br />
družba d.d.,<br />
Verovškova 57,<br />
Ljubljana, Republic of<br />
Slovenia<br />
Lek farmacevtska<br />
družba d.d., Ljubljana,<br />
Republic of Slovenia<br />
Lek farmacevtska<br />
družba d.d.,<br />
Verovškova 57,<br />
Ljubljana, Republic of<br />
Slovenia<br />
Lek farmacevtska<br />
družba d.d., Ljubljana,<br />
Republic of Slovenia<br />
Pliva <strong>Croatia</strong> Ltd., Ulica<br />
grada Vukovara 49,<br />
Zagreb, Republic of<br />
<strong>Croatia</strong><br />
28 (4x7) suppositories in an<br />
aluminium blister, supplied in<br />
a box<br />
7 individually packed<br />
containers (in bags) with 100<br />
mL of suspension <strong>and</strong> an<br />
application attachment, in a<br />
protective bag <strong>and</strong> 7 plastic<br />
hygiene bags, supplied in a<br />
box<br />
100 (10x10) tablets in<br />
auminum blisters, supplied in<br />
a box<br />
Box with a glass bottle with<br />
powder <strong>and</strong> a glass syringe<br />
with 0.5 mL of suspension<br />
with a needle <strong>and</strong> a needle<br />
protection<br />
5 mL of solution for injection<br />
in a glass ampoule, 5<br />
ampoules in a box<br />
20 (2x10) prolonged-release<br />
tablets in PVC/Al blister,<br />
supplied in a box<br />
10 (1x10) film coated tablets<br />
in a OPA/Al/PVC//Al blister,<br />
supplied in a box<br />
One glass vial with 100 mL of<br />
solution for infusion, 12 vials<br />
in a transparent protective<br />
container (polyethylene with<br />
<strong>the</strong>rmo film)<br />
One glass vial with 50 mL of<br />
solution for infusion, 12 vials<br />
in a transparent protective<br />
container (polyethylene with<br />
<strong>the</strong>rmo film)<br />
50 coated tablets in a white<br />
plastic container (PP) with a<br />
white stopper (PE), supplied<br />
in a box<br />
Box with 40 sugar coated<br />
tablets (blister, 4x10 sugar<br />
coated tablets)<br />
20 (2x10) capsules in a<br />
(PVC/PCTFE//Al) blister,<br />
supplied in a box<br />
Box with 20 capsules (blister,<br />
2x10 capsules)<br />
40 (4x10) coated tablets in a<br />
blister (PVC/TE/PVDC//Al),<br />
supplied in a box<br />
5 ampoules with powder <strong>and</strong><br />
5 ampoules each with 2 mL<br />
of solvent (water for<br />
injection), supplied in a box<br />
YES YES<br />
YES YES<br />
YES YES<br />
14509/11 WS/jj 349<br />
ANNEX II DGF 2A / DG K 2 EN<br />
YES<br />
YES YES<br />
YES YES<br />
YES YES YES<br />
YES YES YES YES<br />
YES YES YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES<br />
YES YES