Download - Evonik Industries
Download - Evonik Industries
Download - Evonik Industries
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
it Remains a challenge for catalysis experts to completely<br />
characterize a solid catalyst. Due to the complexity<br />
of these catalysts, they do not form a uniform<br />
phase, but display various polymorphic solid phases<br />
at once, which are also not defined at the molecular<br />
level. As a result, many physical and chemical properties<br />
are subject to broad distribution. Good examples<br />
include the particle sizes of the carrier materials,<br />
the size of the metal particles on the carriers, the<br />
acidity or basicity of surface centers, or even the oxidation<br />
conditions of the active transition metal components.<br />
It becomes even more complicated when the conditions<br />
on the edges of the distribution functions influence<br />
the catalytic properties. With their content<br />
being so infinitesimally small, it is difficult or impossible<br />
to use physicochemical methods to identify and<br />
quantify them.<br />
As a catalyst producer, however, <strong>Evonik</strong> must ensure<br />
the consistent quality and performance of its<br />
products. In addition to the standard methods of physicochemical<br />
characterization, such as determining<br />
particle sizes, elementary analysis, determining pore<br />
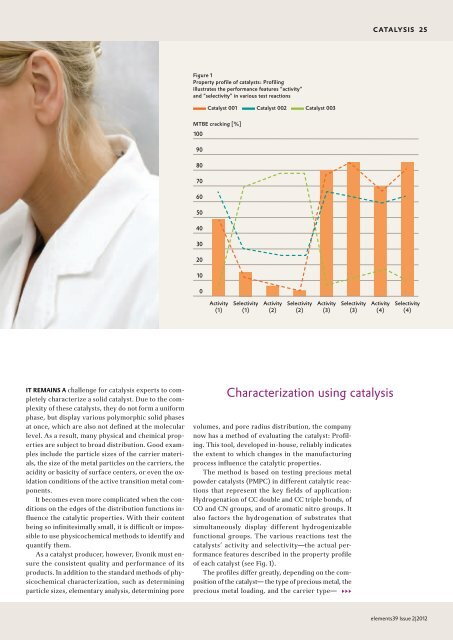
Figure 1<br />
Property profile of catalysts: Profiling<br />
illustrates the performance features “activity”<br />
and “selectivity” in various test reactions<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
Catalyst 001 Catalyst 002 Catalyst 003<br />
MtBe cracking [%]<br />
0<br />
Activity<br />
(1)<br />
Selectivity<br />
(1)<br />
Activity<br />
(2)<br />
Selectivity<br />
(2)<br />
Activity<br />
(3)<br />
Selectivity<br />
(3)<br />
Characterization using catalysis<br />
volumes, and pore radius distribution, the company<br />
now has a method of evaluating the catalyst: Profiling.<br />
This tool, developed in-house, reliably indicates<br />
the extent to which changes in the manufacturing<br />
process influence the catalytic properties.<br />
The method is based on testing precious metal<br />
powder catalysts (PMPC) in different catalytic reactions<br />
that represent the key fields of application:<br />
Hydrogenation of CC double and CC triple bonds, of<br />
CO and CN groups, and of aromatic nitro groups. It<br />
also factors the hydrogenation of substrates that<br />
simultaneously display different hydrogenizable<br />
functional groups. The various reactions test the<br />
catalysts’ activity and selectivity—the actual performance<br />
features described in the property profile<br />
of each catalyst (see Fig. 1).<br />
The profiles differ greatly, depending on the composition<br />
of the catalyst— the type of precious metal, the<br />
precious metal loading, and the carrier type— 333<br />
CAtALYsIs<br />
Activity<br />
(4)<br />
Selectivity<br />
(4)<br />
25<br />
elements39 Issue 2|2012