hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
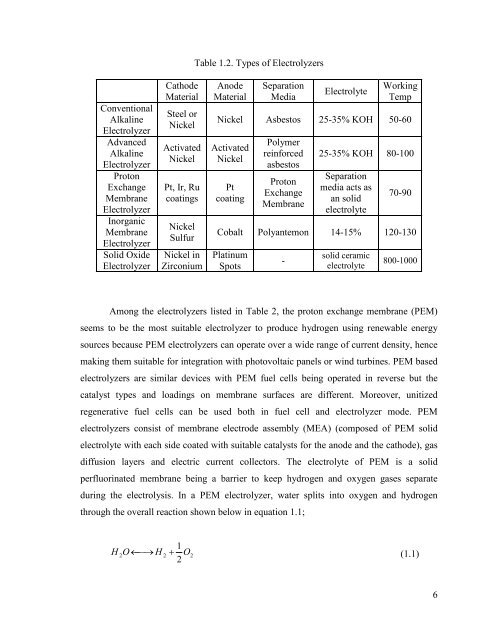
Conventional<br />
Alkaline<br />
Electrolyzer<br />
Advanced<br />
Alkaline<br />
Electrolyzer<br />
Proton<br />
Exchange<br />
Membrane<br />
Electrolyzer<br />
Inorganic<br />
Membrane<br />
Electrolyzer<br />
Solid Oxide<br />
Electrolyzer<br />
Cathode<br />
Material<br />
Steel or<br />
Nickel<br />
Activated<br />
Nickel<br />
Pt, Ir, Ru<br />
coatings<br />
Nickel<br />
Sulfur<br />
Nickel in<br />
Zirconium<br />
Table 1.2. Types of Electrolyzers<br />
Anode<br />
Material<br />
Separation<br />
Media<br />
Electrolyte<br />
Working<br />
Temp<br />
Nickel Asbestos 25-35% KOH 50-60<br />
Activated<br />
Nickel<br />
Pt<br />
coating<br />
Polymer<br />
reinforced<br />
asbestos<br />
Proton<br />
Exchange<br />
Membrane<br />
25-35% KOH 80-100<br />
Separation<br />
media acts as<br />
an solid<br />
electrolyte<br />
70-90<br />
Cobalt Polyantemon 14-15% 120-130<br />
Platinum<br />
Spots<br />
-<br />
solid ceramic<br />
electrolyte<br />
800-1000<br />
Among the electrolyzers listed in Table 2, the proton exchange membrane (PEM)<br />
seems to be the most suitable electrolyzer to produce <strong>hydrogen</strong> <strong>using</strong> renewable energy<br />
sources because PEM electrolyzers can operate over a wide range of current density, hence<br />
making them suitable for integration with photovoltaic panels or wind turbines. PEM based<br />
electrolyzers are similar devices with PEM fuel <strong>cells</strong> being operated in reverse but the<br />
catalyst types and loadings on membrane surfaces are different. Moreover, unitized<br />
regenerative fuel <strong>cells</strong> can be used both in fuel cell and electrolyzer mode. PEM<br />
electrolyzers consist of membrane electrode assembly (MEA) (composed of PEM solid<br />
electrolyte with each side coated with suitable catalysts for the anode and the cathode), gas<br />
diffusion layers and electric current collectors. The electrolyte of PEM is a solid<br />
perfluorinated membrane being a barrier to keep <strong>hydrogen</strong> and oxygen gases separate<br />
during the electrolysis. In a PEM electrolyzer, <strong>water</strong> splits into oxygen and <strong>hydrogen</strong><br />
through the overall reaction shown below in equation 1.1;<br />
1<br />
H 2O<br />
⎯→ + O<br />
2<br />
← H 2 2<br />
(1.1)<br />
6