hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
(which is usually the case for photovoltaic <strong>powered</strong> electrolysis) <strong>hydrogen</strong> back diffusion<br />
results with an explosive gas output on the oxygen line. To avoid that N-117 was selected<br />
as proton exchange membrane for this experimental setup.<br />
4.3. Results on a Five cell PEM Electrolyzer Stack<br />
A five cell electrolysis stack was constructed according to the procedure as<br />
mentioned in the previous chapter. First, the stack was tested with a regulated DC current<br />
supply to examine if there is any problem, such as gas mixing, <strong>water</strong> distribution or voltage<br />
distribution between the <strong>cells</strong> of the stack.<br />
The <strong>water</strong> flow was set to 10g/min and also external fans were used depending on<br />
the current densities so that it was easy to keep the stack temperature at 40 o C. The supplied<br />
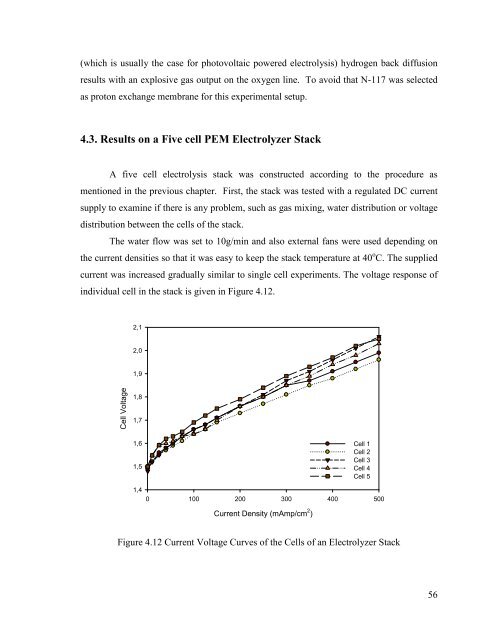
current was increased gradually similar to single cell experiments. The voltage response of<br />
individual cell in the stack is given in Figure 4.12.<br />
Cell Voltage<br />
2,1<br />
2,0<br />
1,9<br />
1,8<br />
1,7<br />
1,6<br />
1,5<br />
1,4<br />
0 100 200 300 400 500<br />
Current Density (mAmp/cm 2 )<br />
Cell 1<br />
Cell 2<br />
Cell 3<br />
Cell 4<br />
Cell 5<br />
Figure 4.12 Current Voltage Curves of the Cells of an Electrolyzer Stack<br />
56