hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
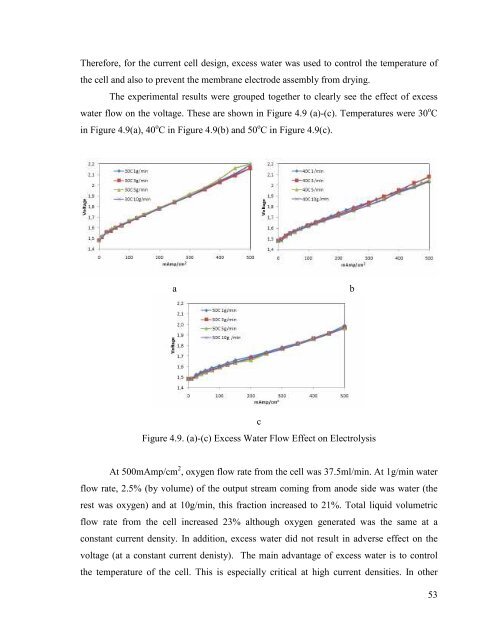
Therefore, for the current cell design, excess <strong>water</strong> was used to control the temperature of<br />
the cell and also to prevent the membrane electrode assembly <strong>from</strong> drying.<br />
The experimental results were grouped together to clearly see the effect of excess<br />
<strong>water</strong> flow on the voltage. These are shown in Figure 4.9 (a)-(c). Temperatures were 30 o C<br />
in Figure 4.9(a), 40 o C in Figure 4.9(b) and 50 o C in Figure 4.9(c).<br />
a b<br />
c<br />
Figure 4.9. (a)-(c) Excess Water Flow Effect on Electrolysis<br />
At 500mAmp/cm 2 , oxygen flow rate <strong>from</strong> the cell was 37.5ml/min. At 1g/min <strong>water</strong><br />
flow rate, 2.5% (by volume) of the output stream coming <strong>from</strong> anode side was <strong>water</strong> (the<br />
rest was oxygen) and at 10g/min, this fraction increased to 21%. Total liquid volumetric<br />
flow rate <strong>from</strong> the cell increased 23% although oxygen generated was the same at a<br />
constant current density. In addition, excess <strong>water</strong> did not result in adverse effect on the<br />
voltage (at a constant current denisty). The main advantage of excess <strong>water</strong> is to control<br />
the temperature of the cell. This is especially critical at high current densities. In other<br />
53