hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
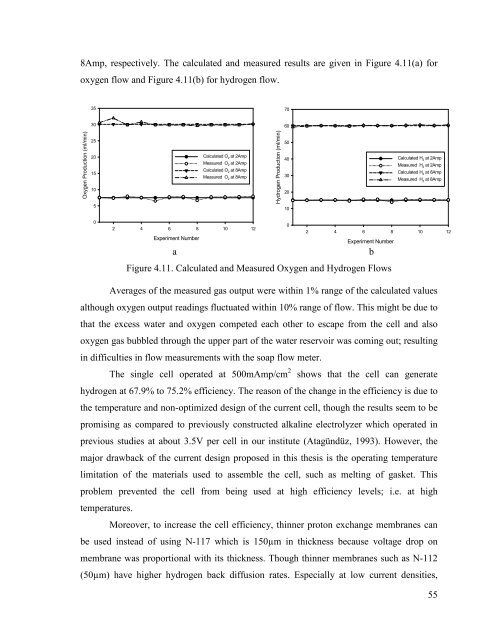
8Amp, respectively. The calculated and measured results are given in Figure 4.11(a) for<br />
oxygen flow and Figure 4.11(b) for <strong>hydrogen</strong> flow.<br />
Oxygen Production (ml/min)<br />
35<br />
30<br />
25<br />
20<br />
15<br />
10<br />
5<br />
0<br />
a b<br />
Figure 4.11. Calculated and Measured Oxygen and Hydrogen Flows<br />
Averages of the measured gas output were within 1% range of the calculated values<br />
although oxygen output readings fluctuated within 10% range of flow. This might be due to<br />
that the excess <strong>water</strong> and oxygen competed each other to escape <strong>from</strong> the cell and also<br />
oxygen gas bubbled through the upper part of the <strong>water</strong> reservoir was coming out; resulting<br />
in difficulties in flow measurements with the soap flow meter.<br />
The single cell operated at 500mAmp/cm 2 shows that the cell can generate<br />
<strong>hydrogen</strong> at 67.9% to 75.2% efficiency. The reason of the change in the efficiency is due to<br />
the temperature and non-optimized design of the current cell, though the results seem to be<br />
promising as compared to previously constructed alkaline electrolyzer which operated in<br />
previous studies at about 3.5V per cell in our institute (Atagündüz, 1993). However, the<br />
major drawback of the current design proposed in this thesis is the operating temperature<br />
limitation of the materials used to assemble the cell, such as melting of gasket. This<br />
problem prevented the cell <strong>from</strong> being used at high efficiency levels; i.e. at high<br />
temperatures.<br />
2 4 6 8 10 12<br />
Experiment Number<br />
Calculated O 2 at 2Amp<br />
Measured O 2 at 2Amp<br />
Calculated O 2 at 8Amp<br />
Measured O 2 at 8Amp<br />
Moreover, to increase the cell efficiency, thinner proton exchange membranes can<br />
be used instead of <strong>using</strong> N-117 which is 150µm in thickness because voltage drop on<br />
membrane was proportional with its thickness. Though thinner membranes such as N-112<br />
(50µm) have higher <strong>hydrogen</strong> back diffusion rates. Especially at low current densities,<br />
Hydrogen Production (ml/min)<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0<br />
2 4 6 8 10 12<br />
Experiment Number<br />
Calculated H 2 at 2Amp<br />
Measured H 2 at 2Amp<br />
Calculated H 2 at 8Amp<br />
Measured H 2 at 8Amp<br />
55