hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.2. Electrolyzers<br />
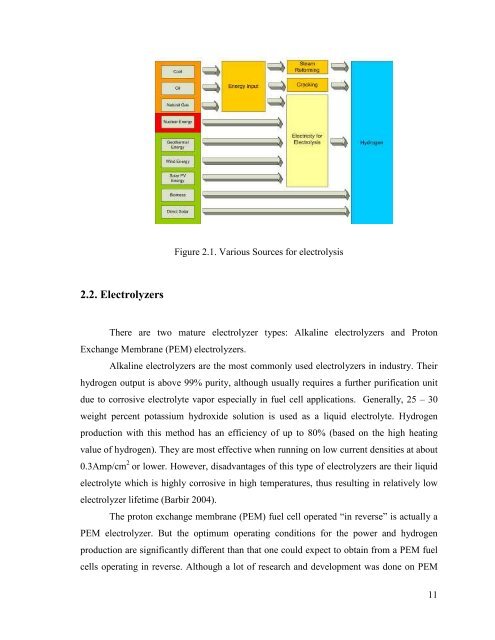
Figure 2.1. Various Sources for electrolysis<br />
There are two mature electrolyzer types: Alkaline electrolyzers and Proton<br />
Exchange Membrane (PEM) electrolyzers.<br />
Alkaline electrolyzers are the most commonly used electrolyzers in industry. Their<br />
<strong>hydrogen</strong> output is above 99% purity, although usually requires a further purification unit<br />
due to corrosive electrolyte vapor especially in fuel cell applications. Generally, 25 – 30<br />
weight percent potassium hydroxide solution is used as a liquid electrolyte. Hydrogen<br />
<strong>production</strong> with this method has an efficiency of up to 80% (based on the high heating<br />
value of <strong>hydrogen</strong>). They are most effective when running on low current densities at about<br />
0.3Amp/cm 2 or lower. However, disadvantages of this type of electrolyzers are their liquid<br />
electrolyte which is highly corrosive in high temperatures, thus resulting in relatively low<br />
electrolyzer lifetime (Barbir 2004).<br />
The proton exchange membrane (PEM) fuel cell operated “in reverse” is actually a<br />
PEM electrolyzer. But the optimum operating conditions for the power and <strong>hydrogen</strong><br />
<strong>production</strong> are significantly different than that one could expect to obtain <strong>from</strong> a PEM fuel<br />
<strong>cells</strong> operating in reverse. Although a lot of research and development was done on PEM<br />
11