hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
hydrogen production from water using solar cells powered nafion ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Different than Grigoriev’s work, Yim and coworkers tried to make regenerative<br />
electrolyzers by adding Pt into anode electrode. Regenerative electrolyzer (or fuel cell) can<br />
generate oxygen and <strong>hydrogen</strong> when electricity available or can generate electricity when<br />
oxygen and <strong>hydrogen</strong> is available. In order to simplify the comparison between different<br />
anode catalyst loadings, 4.0mg/cm 2 Pt loaded cathode is used in all the experiments (Yim et<br />
al 2004). The catalyst types and their loadings are listed in Table 2.2.<br />
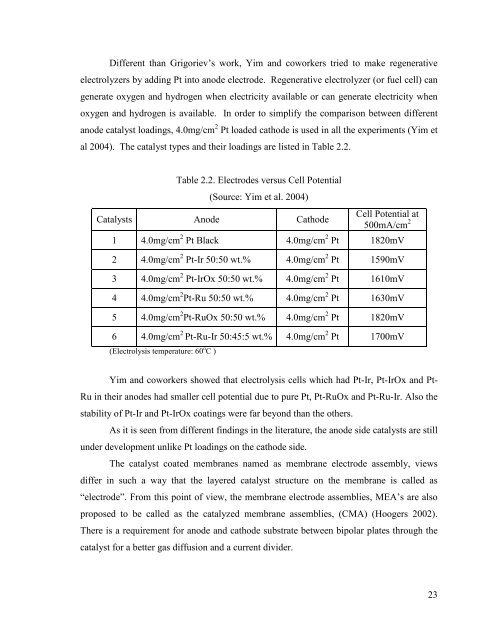
Table 2.2. Electrodes versus Cell Potential<br />
(Source: Yim et al. 2004)<br />
Catalysts Anode Cathode<br />
Cell Potential at<br />
500mA/cm 2<br />
1 4.0mg/cm 2 Pt Black 4.0mg/cm 2 Pt 1820mV<br />
2 4.0mg/cm 2 Pt-Ir 50:50 wt.% 4.0mg/cm 2 Pt 1590mV<br />
3 4.0mg/cm 2 Pt-IrOx 50:50 wt.% 4.0mg/cm 2 Pt 1610mV<br />
4 4.0mg/cm 2 Pt-Ru 50:50 wt.% 4.0mg/cm 2 Pt 1630mV<br />
5 4.0mg/cm 2 Pt-RuOx 50:50 wt.% 4.0mg/cm 2 Pt 1820mV<br />
6 4.0mg/cm 2 Pt-Ru-Ir 50:45:5 wt.% 4.0mg/cm 2 Pt 1700mV<br />
(Electrolysis temperature: 60 o C )<br />
Yim and coworkers showed that electrolysis <strong>cells</strong> which had Pt-Ir, Pt-IrOx and Pt-<br />
Ru in their anodes had smaller cell potential due to pure Pt, Pt-RuOx and Pt-Ru-Ir. Also the<br />
stability of Pt-Ir and Pt-IrOx coatings were far beyond than the others.<br />
As it is seen <strong>from</strong> different findings in the literature, the anode side catalysts are still<br />
under development unlike Pt loadings on the cathode side.<br />
The catalyst coated membranes named as membrane electrode assembly, views<br />
differ in such a way that the layered catalyst structure on the membrane is called as<br />
“electrode”. From this point of view, the membrane electrode assemblies, MEA’s are also<br />
proposed to be called as the catalyzed membrane assemblies, (CMA) (Hoogers 2002).<br />
There is a requirement for anode and cathode substrate between bipolar plates through the<br />
catalyst for a better gas diffusion and a current divider.<br />
23