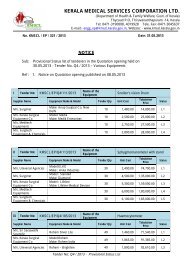
empanelment of drug testing laboratories - Kerala Medical Services ...
empanelment of drug testing laboratories - Kerala Medical Services ...
empanelment of drug testing laboratories - Kerala Medical Services ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
5.7.4 It is also an opportunity for the Corporation to assess the market and<br />
obtain feedback on the current developments in <strong>drug</strong> <strong>testing</strong>, so as to<br />
make amendments in the EOI document on the basis <strong>of</strong> expert advice.<br />
5.7.5 All prospective <strong>of</strong>ferers can attend the pre <strong>of</strong>fer meeting. The venue, date<br />
and time is indicated in Section III.<br />
5.7.6 Failure to attend the pre-<strong>of</strong>fer meeting will not be a disqualification, but a<br />
loss <strong>of</strong> opportunity for the prospective <strong>of</strong>ferers to understand the EOI<br />
conditions.<br />
5.7.7 Filled up EOI will be accepted only after the date <strong>of</strong> pre <strong>of</strong>fer meeting.<br />
5.8 Amendment <strong>of</strong> EOI documents:<br />
5.8.1 At any time prior to the dead line for submission <strong>of</strong> EOI, the EOI Inviting<br />
Authority may, for any reason, modify the EOI document by<br />
amendment.<br />
5.8.2 The amendment shall be published in the website <strong>of</strong> the Corporation or<br />
notified by fax/email to all prospective <strong>of</strong>ferers who have purchased the<br />
EOI document, for which the email, fax no <strong>of</strong> the purchaser <strong>of</strong> the EOI<br />
document shall be submitted to the EOI inviting authority and such<br />
amendments, shall be binding on them thereafter.<br />
5.8.3 The Corporation shall not be responsible for failure to inform the<br />
prospective <strong>of</strong>ferers because <strong>of</strong> technical issues, wrong fax number or<br />
email ID etc. Purchasers <strong>of</strong> EOI documents are requested to browse the<br />
website <strong>of</strong> the Corporation for information / general notices<br />
/amendments to EOI document etc on a day to day basis till the EOI is<br />
concluded.<br />
5.9 EOI Process<br />
The EOI Process is divided into two:<br />
5.9.1 Scrutiny <strong>of</strong> technical documents submitted before the schedule date and<br />
time.<br />
5.9.2 Inspection <strong>of</strong> the shortlisted lab by a technical committee on behalf <strong>of</strong> the<br />
Corporation and selection <strong>of</strong> the lab.<br />
5.10 Contents <strong>of</strong> the EOI documents<br />
5.10.1 The <strong>of</strong>ferer must submit the following documents in a sealed cover<br />
5.10.2 Checklist (Annexure –I) for the list <strong>of</strong> documents enclosed with their page<br />
Nos. The documents should be serially numbered and arranged as per<br />
KMSCL: EOI for Empanelment <strong>of</strong> Drug <strong>testing</strong> Laboratories Page 14