Report in English with a Dutch summary (KCE reports 63A)
Report in English with a Dutch summary (KCE reports 63A)
Report in English with a Dutch summary (KCE reports 63A)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
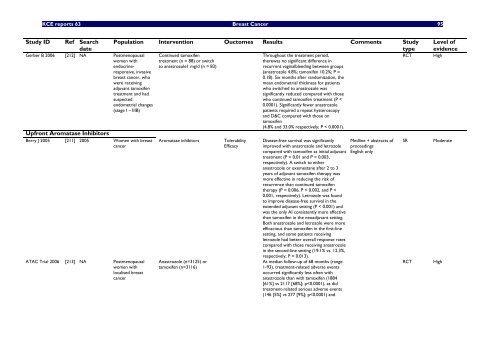
<strong>KCE</strong> <strong>reports</strong> 63 Breast Cancer 95<br />
Study ID Ref Search<br />
date<br />
Gerber B 2006 [212] NA Postmenopausal<br />
women <strong>with</strong><br />
endocr<strong>in</strong>eresponsive,<br />
<strong>in</strong>vasive<br />
breast cancer, who<br />
were receiv<strong>in</strong>g<br />
adjuvant tamoxifen<br />
treatment and had<br />
suspected<br />
endometrial changes<br />
(stage I – IIIB)<br />
Upfront Aromatase Inhibitors<br />
Berry J 2005 [211] 2005 Women <strong>with</strong> breast<br />
cancer<br />
ATAC Trial 2006 [213] NA Postmenopausal<br />
women <strong>with</strong><br />
localised breast<br />
cancer<br />
Population Intervention Ouctomes Results Comments Study<br />
type<br />
Cont<strong>in</strong>ued tamoxifen<br />
treatment (n = 88) or switch<br />
to anastrozole1 mg/d (n = 83)<br />
Aromatase <strong>in</strong>hibitors Tolerability<br />
Efficacy<br />
Anastrozole (n=3125) or<br />
tamoxifen (n=3116)<br />
Throughout the treatment period,<br />
therewas no significant difference <strong>in</strong><br />
recurrent vag<strong>in</strong>albleed<strong>in</strong>g between groups<br />
(anastrozole 4.8%; tamoxifen 10.2%; P =<br />
0.18). Six months after randomization, the<br />
mean endometrial thickness for patients<br />
who switched to anastrozole was<br />
significantly reduced compared <strong>with</strong> those<br />
who cont<strong>in</strong>ued tamoxifen treatment (P <<br />
0.0001). Significantly fewer anastrozole<br />
patients required a repeat hysteroscopy<br />
and D&C compared <strong>with</strong> those on<br />
tamoxifen<br />
(4.8% and 33.0% respectively; P < 0.0001).<br />
Disease-free survival was significantly<br />
improved <strong>with</strong> anastrozole and letrozole<br />
compared <strong>with</strong> tamoxifen as <strong>in</strong>itial adjuvant<br />
treatment (P = 0.01 and P = 0.003,<br />
respectively). A switch to either<br />
anastrozole or exemestane after 2 to 3<br />
years of adjuvant tamoxifen therapy was<br />
more effective <strong>in</strong> reduc<strong>in</strong>g the risk of<br />
recurrence than cont<strong>in</strong>ued tamoxifen<br />
therapy (P = 0.006, P < 0.002, and P <<br />
0.001, respectively). Letrozole was found<br />
to improve disease-free survival <strong>in</strong> the<br />
extended adjuvant sett<strong>in</strong>g (P < 0.001) and<br />
was the only AI consistently more effective<br />
than tamoxifen <strong>in</strong> the neoadjuvant sett<strong>in</strong>g.<br />
Both anastrozole and letrozole were more<br />
efficacious than tamoxifen <strong>in</strong> the first-l<strong>in</strong>e<br />
sett<strong>in</strong>g, and some patients receiv<strong>in</strong>g<br />
letrozole had better overall response rates<br />
compared <strong>with</strong> those receiv<strong>in</strong>g anastrozole<br />
<strong>in</strong> the second-l<strong>in</strong>e sett<strong>in</strong>g (19.1% vs. 12.3%,<br />
respectively; P = 0.013).<br />
At median follow-up of 68 months (range<br />
1-93), treatment-related adverse events<br />
occurred significantly less often <strong>with</strong><br />
anastrozole than <strong>with</strong> tamoxifen (1884<br />
[61%] vs 2117 [68%]; p