Report in English with a Dutch summary (KCE reports 63A)
Report in English with a Dutch summary (KCE reports 63A)
Report in English with a Dutch summary (KCE reports 63A)
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
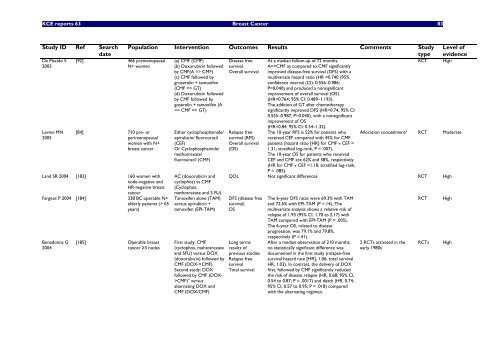
<strong>KCE</strong> <strong>reports</strong> 63 Breast Cancer 83<br />
Study ID Ref Search<br />
date<br />
De Placido S<br />
2005<br />
Lev<strong>in</strong>e MN<br />
2005<br />
[92] 466 premenopausal<br />
N+ women<br />
[84] 710 pre- or<br />
perimenopausal<br />
women <strong>with</strong> N+<br />
breast cancer<br />
Land SR 2004 [183] 160 women <strong>with</strong><br />
node-negative and<br />
HR-negative breast<br />
Population Intervention Outcomes Results Comments Study<br />
type<br />
cancer<br />
Fargeot P 2004 [184] 338 BC operable N+<br />
elderly patients (> 65<br />
years)<br />
Bonadonna G<br />
2004<br />
[185] Operable breast<br />
cancer ≥3 nodes<br />
(a) CMF (CMF)<br />
(b) Doxorubic<strong>in</strong> followed<br />
by CMF(A => CMF)<br />
(c) CMF followed by<br />
groserel<strong>in</strong> + tamoxifen<br />
(CMF => GT)<br />
(d) Doxorubic<strong>in</strong> followed<br />
by CMF followed by<br />
goserel<strong>in</strong> + tamoxifen (A<br />
=> CMF => GT)<br />
Either cyclophosphamide/<br />
epirubic<strong>in</strong>/ fluorouracil<br />
(CEF)<br />
Or Cyclophosphamide/<br />
methotrexate/<br />
fluorouracil (CMF)<br />
AC (doxorubic<strong>in</strong> and<br />
cyclophos) vs CMF<br />
(Cyclophos,<br />
methotrexate and 5 FU)<br />
Tamoxifen alone (TAM)<br />
versus epirubic<strong>in</strong> +<br />
tamoxifen (EPI-TAM)<br />
First study: CMF<br />
(cyclophos, mehtotrexate<br />
and 5FU) versus DOX<br />
(doxorubic<strong>in</strong>) followed by<br />
CMF (DOX->CMF)<br />
Second study: DOX<br />
followed by CMF (DOX-<br />
>CMF)° versus<br />
alternat<strong>in</strong>g DOX and<br />
CMF (DOX/CMF)<br />
Disease free<br />
survival<br />
Overall survival<br />
Relapse free<br />
survival (RFS)<br />
Overall survival<br />
(OS)<br />
At a median follow-up of 72 months,<br />
A=>CMF as compared to CMF significantly<br />
improved disease-free survival (DFS) <strong>with</strong> a<br />
multivariate hazard ratio (HR =0.740 (95%<br />
confidence <strong>in</strong>terval (CI): 0.556–0.986;<br />
P=0.040) and produced a nonsignificant<br />
improvement of overall survival (OS)<br />
(HR=0.764; 95% CI: 0.489–1.193).<br />
The addition of GT after chemotherapy<br />
significantly improved DFS (HR=0.74; 95% CI:<br />
0.555–0.987; P=0.040), <strong>with</strong> a nonsignificant<br />
improvement of OS<br />
(HR=0.84; 95% CI: 0.54–1.32).<br />
The 10-year RFS is 52% for patients who<br />
received CEF compared <strong>with</strong> 45% for CMF<br />
patients (hazard ratio [HR] for CMF v CEF =<br />
1.31; stratified log-rank, P = .007).<br />
The 10-year OS for patients who received<br />
CEF and CMF are 62% and 58%, respectively<br />
(HR for CMF v CEF =1.18; stratified log-rank,<br />
P = .085).<br />
RCT High<br />
Level of<br />
evidence<br />
Allocation concealment? RCT Moderate<br />
QOL Not significant differences RCT High<br />
DFS (disease free<br />
survival)<br />
OS<br />
Long terms<br />
results of<br />
previous studies<br />
Relapse free<br />
survival<br />
Total survival<br />
The 6-year DFS rates were 69.3% <strong>with</strong> TAM<br />
and 72.6% <strong>with</strong> EPI-TAM (P =.14). The<br />
multivariate analysis shows a relative risk of<br />
relapse of 1.93 (95% CI, 1.70 to 2.17) <strong>with</strong><br />
TAM compared <strong>with</strong> EPI-TAM (P = .005).<br />
The 6-year OS, related to disease<br />
progression, was 79.1% and 79.8%,<br />
respectively (P =.41).<br />
After a median observation of 210 months,<br />
no statistically significant difference was<br />
documented <strong>in</strong> the first study (relapse-free<br />
survival hazard rate [HR], 1.06; total survival<br />
HR, 1.03). In contrast, the delivery of DOX<br />
first, followed by CMF significantly reduced<br />
the risk of disease relapse (HR, 0.68; 95% CI,<br />
0.54 to 0.87; P = .0017) and death (HR, 0.74;<br />
95% CI, 0.57 to 0.95; P = .018) compared<br />
<strong>with</strong> the alternat<strong>in</strong>g regimen.<br />
2 RCTs activated <strong>in</strong> the<br />
early 1980s<br />
RCT<br />
High<br />
RCTs High