Report in English with a Dutch summary (KCE reports 63A)
Report in English with a Dutch summary (KCE reports 63A)
Report in English with a Dutch summary (KCE reports 63A)
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
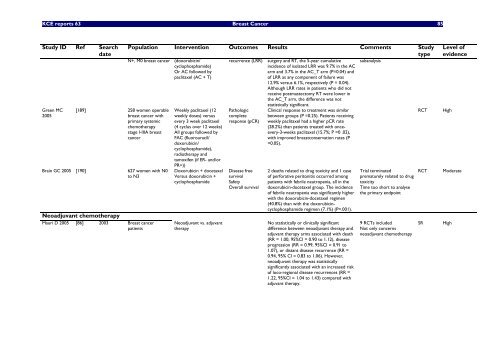
<strong>KCE</strong> <strong>reports</strong> 63 Breast Cancer 85<br />
Study ID Ref Search<br />
date<br />
Green MC<br />
2005<br />
[189] 258 women operable<br />
breast cancer <strong>with</strong><br />
primary systemic<br />
chemotherapy<br />
stage I-IIIA breast<br />
cancer<br />
Bra<strong>in</strong> GC 2005 [190] 627 women <strong>with</strong> N0<br />
to N3<br />
Neoadjuvant chemotherapy<br />
Mauri D 2005 [86] 2003 Breast cancer<br />
patients<br />
Population Intervention Outcomes Results Comments Study<br />
type<br />
N+, M0 breast cancer (doxorubic<strong>in</strong>/<br />
cyclophosphamide)<br />
Or AC followed by<br />
paclitaxel (AC + T)<br />
Weekly paclitaxel (12<br />
weekly doses) versus<br />
every 3 week paclitaxel<br />
(4 cyclus over 12 weeks)<br />
All groups followed by<br />
FAC (fluorouracil/<br />
doxorubic<strong>in</strong>/<br />
cyclophosphamide),<br />
radiotherapy and<br />
tamoxifen (if ER- and/or<br />
PR+))<br />
Doxorubic<strong>in</strong> + docetaxel<br />
Versus doxorubic<strong>in</strong> +<br />
cyclophosphamide<br />
Neoadjuvant vs. adjuvant<br />
therapy<br />
recurrence (LRR)<br />
Pathologic<br />
complete<br />
response (pCR)<br />
Disease free<br />
survival<br />
Safety<br />
Overall survival<br />
surgery and RT, the 5-year cumulative<br />
<strong>in</strong>cidence of isolated LRR was 9.7% <strong>in</strong> the AC<br />
arm and 3.7% <strong>in</strong> the AC_T arm (P=0.04) and<br />
of LRR as any component of failure was<br />
12.9% versus 6.1%, respectively (P = 0.04).<br />
Although LRR rates <strong>in</strong> patients who did not<br />
receive postmastectomy RT were lower <strong>in</strong><br />
the AC_T arm, the difference was not<br />
statistically significant.<br />
Cl<strong>in</strong>ical response to treatment was similar<br />
between groups (P =0.25). Patients receiv<strong>in</strong>g<br />
weekly paclitaxel had a higher pCR rate<br />
(28.2%) than patients treated <strong>with</strong> onceevery-3-weeks<br />
paclitaxel (15.7%; P =0 .02),<br />
<strong>with</strong> improved breastconservation rates (P<br />
=0.05).<br />
2 deaths related to drug toxicity and 1 case<br />
of perforative peritonitis occurred among<br />
patients <strong>with</strong> febrile neutropenia, all <strong>in</strong> the<br />
doxorubic<strong>in</strong>-docetaxel group. The <strong>in</strong>cidence<br />
of febrile neutropenia was significantly higher<br />
<strong>with</strong> the doxorubic<strong>in</strong>-docetaxel regimen<br />
(40.8%) than <strong>with</strong> the doxorubic<strong>in</strong>cyclophosphamide<br />
regimen (7.1%) (P=.001).<br />
No statistically or cl<strong>in</strong>ically significant<br />
difference between neoadjuvant therapy and<br />
adjuvant therapy arms associated <strong>with</strong> death<br />
(RR = 1.00, 95%CI = 0.90 to 1.12), disease<br />
progression (RR = 0.99, 95%CI = 0.91 to<br />
1.07), or distant disease recurrence (RR =<br />
0.94, 95% CI = 0.83 to 1.06). However,<br />
neoadjuvant therapy was statistically<br />
significantly associated <strong>with</strong> an <strong>in</strong>creased risk<br />
of loco-regional disease recurrences (RR =<br />
1.22, 95%CI = 1.04 to 1.43) compared <strong>with</strong><br />
adjuvant therapy.<br />
subanalysis<br />
Trial term<strong>in</strong>ated<br />
prematurely related to drug<br />
toxicity<br />
Time too short to analyse<br />
the primary endpo<strong>in</strong>t<br />
9 RCTs <strong>in</strong>cluded<br />
Not only concerns<br />
neoadjuvant chemotherapy<br />
RCT High<br />
Level of<br />
evidence<br />
RCT Moderate<br />
SR High