Thesis for the Degree of Doctor of Philosophy - DTU Orbit
Thesis for the Degree of Doctor of Philosophy - DTU Orbit
Thesis for the Degree of Doctor of Philosophy - DTU Orbit
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
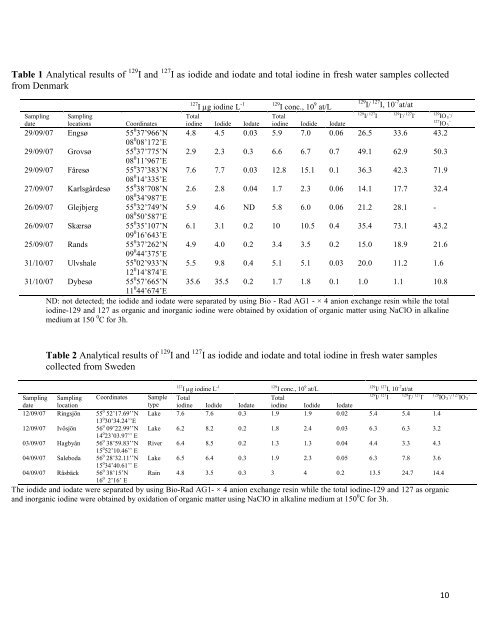
Table 1 Analytical results <strong>of</strong> 129 I and 127 I as iodide and iodate and total iodine in fresh water samples collected<br />
from Denmark<br />
127 -1<br />
I µg iodine L<br />
129 9<br />
I conc., 10 at/L<br />
129 127 -7<br />
I/ I, 10 at/at<br />
Sampling<br />
date<br />
Sampling<br />
locations Coordinates<br />
Total<br />
iodine Iodide Iodate<br />
Total<br />
iodine Iodide Iodate<br />
129 127<br />
I/ I<br />
129 - 127 -<br />
I / I<br />
129 -<br />
IO 3 /<br />
127 -<br />
IO 3<br />
29/09/07 Engsø 55 0 37’966’N<br />
08 0 08’172’E<br />
4.8 4.5 0.03 5.9 7.0 0.06 26.5 33.6 43.2<br />
29/09/07 Grovsø 55 0 37’775’N<br />
08 0 11’967’E<br />
2.9 2.3 0.3 6.6 6.7 0.7 49.1 62.9 50.3<br />
29/09/07 Fåresø 55 0 37’383’N<br />
08 0 14’335’E<br />
7.6 7.7 0.03 12.8 15.1 0.1 36.3 42.3 71.9<br />
27/09/07 Karlsgårdesø 55 0 38’708’N<br />
08 0 34’987’E<br />
2.6 2.8 0.04 1.7 2.3 0.06 14.1 17.7 32.4<br />
26/09/07 Glejbjerg 55 0 32’749’N<br />
08 0 50’587’E<br />
5.9 4.6 ND 5.8 6.0 0.06 21.2 28.1 -<br />
26/09/07 Skærsø 55 0 35’107’N<br />
09 0 16’643’E<br />
6.1 3.1 0.2 10 10.5 0.4 35.4 73.1 43.2<br />
25/09/07 Rands 55 0 37’262’N<br />
09 0 44’375’E<br />
4.9 4.0 0.2 3.4 3.5 0.2 15.0 18.9 21.6<br />
31/10/07 Ulvshale 55 0 02’933’N<br />
12 0 14’874’E<br />
5.5 9.8 0.4 5.1 5.1 0.03 20.0 11.2 1.6<br />
31/10/07 Dybesø 55 0 57’665’N<br />
11 0 44’674’E<br />
35.6 35.5 0.2 1.7 1.8 0.1 1.0 1.1 10.8<br />
ND: not detected; <strong>the</strong> iodide and iodate were separated by using Bio - Rad AG1 - × 4 anion exchange resin while <strong>the</strong> total<br />
iodine-129 and 127 as organic and inorganic iodine were obtained by oxidation <strong>of</strong> organic matter using NaClO in alkaline<br />
medium at 150 0 C <strong>for</strong> 3h.<br />
Table 2 Analytical results <strong>of</strong> 129 I and 127 I as iodide and iodate and total iodine in fresh water samples<br />
collected from Sweden<br />
127 -1<br />
I µg iodine L<br />
129 9<br />
I conc., 10 at/L<br />
129 127 -7<br />
I/ I, 10 at/at<br />
Sampling Sampling Coordinates Sample Total<br />
Total<br />
129 127<br />
I/ I<br />
129 - 127 -<br />
I / I<br />
129 - 127 -<br />
IO 3 / IO 3<br />
date location<br />
type iodine Iodide Iodate iodine Iodide Iodate<br />
12/09/07 Ringsjön 55 0 52’17.69’’N<br />
13 0 30’34.24’’E<br />
Lake 7.6 7.6 0.3 1.9 1.9 0.02 5.4 5.4 1.4<br />
12/09/07 Ivösjön 56 0 09’22.99’’N<br />
14 0 23’03.97’’ E<br />
Lake 6.2 8.2 0.2 1.8 2.4 0.03 6.3 6.3 3.2<br />
03/09/07 Hagbyån 56 0 38’59.83’’N<br />
15 0 52’10.46’’ E<br />
River 6.4 8.5 0.2 1.3 1.3 0.04 4.4 3.3 4.3<br />
04/09/07 Saleboda 56 0 28’32.11’’N<br />
15 0 34’40.61’’ E<br />
Lake 6.5 6.4 0.3 1.9 2.3 0.05 6.3 7.8 3.6<br />
04/09/07 Råsbäck 56 0 38’15’N<br />
16 0 2’16’ E<br />
Rain 4.8 3.5 0.3 3 4 0.2 13.5 24.7 14.4<br />
The iodide and iodate were separated by using Bio-Rad AG1- × 4 anion exchange resin while <strong>the</strong> total iodine-129 and 127 as organic<br />
and inorganic iodine were obtained by oxidation <strong>of</strong> organic matter using NaClO in alkaline medium at 150 0 C <strong>for</strong> 3h.<br />
10