Thesis for the Degree of Doctor of Philosophy - DTU Orbit
Thesis for the Degree of Doctor of Philosophy - DTU Orbit
Thesis for the Degree of Doctor of Philosophy - DTU Orbit
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
9). The results shows that iodine present in solution was nearly quantitatively removed (around 2.5-7.6 % <strong>of</strong><br />
<strong>the</strong> total iodine remains in <strong>the</strong> water phase after batch experiment, Table 9, experiment numbers 3 and 4)<br />
even when <strong>the</strong> concentration <strong>of</strong> natural organic iodine compounds extracted from soils and seaweed were<br />
added to <strong>the</strong> fresh water (Table 9) increase significantly. By contrast when using a mixture <strong>of</strong><br />
K2S2O8/NaClO as an oxidant, relatively high (30 - 40%) amount <strong>of</strong> iodine remains in <strong>the</strong> water phase after<br />
<strong>the</strong> batch experiment (Table 10, experiments numbers 9 and 10).<br />
Due to <strong>the</strong> low amount <strong>of</strong> iodine that remains in <strong>the</strong> water phase after <strong>the</strong> batch experiments (Table 9,<br />
experiments numbers 3-6) we conclude that <strong>the</strong> oxidation <strong>of</strong> iodine organic matter by using K2S2O8<br />
followed by reduction <strong>of</strong> iodine species and precipitation with silver nitrate can be a potential method <strong>for</strong><br />
determination <strong>of</strong> total iodine in freshwater samples. Because more samples can be treated simultaneously,<br />
<strong>the</strong> method is rapid and very suitable <strong>for</strong> field use if needed.<br />
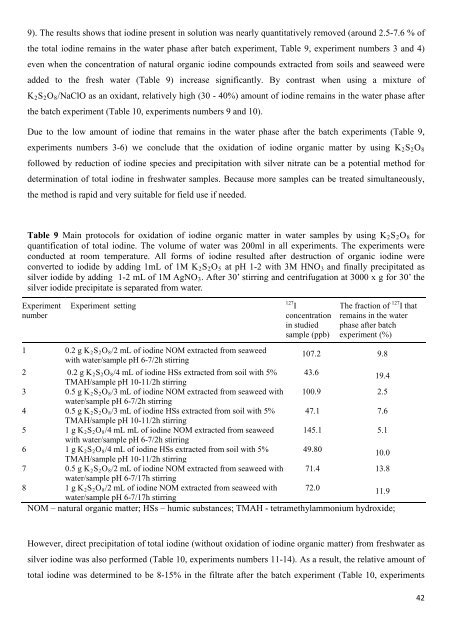
Table 9 Main protocols <strong>for</strong> oxidation <strong>of</strong> iodine organic matter in water samples by using K2S2O8 <strong>for</strong><br />
quantification <strong>of</strong> total iodine. The volume <strong>of</strong> water was 200ml in all experiments. The experiments were<br />
conducted at room temperature. All <strong>for</strong>ms <strong>of</strong> iodine resulted after destruction <strong>of</strong> organic iodine were<br />
converted to iodide by adding 1mL <strong>of</strong> 1M K2S2O5 at pH 1-2 with 3M HNO3 and finally precipitated as<br />
silver iodide by adding 1-2 mL <strong>of</strong> 1M AgNO3. After 30’ stirring and centrifugation at 3000 x g <strong>for</strong> 30’ <strong>the</strong><br />
silver iodide precipitate is separated from water.<br />
Experiment<br />
number<br />
Experiment setting<br />
127 I<br />
concentration<br />
in studied<br />
sample (ppb)<br />
The fraction <strong>of</strong> 127 I that<br />
remains in <strong>the</strong> water<br />
phase after batch<br />
experiment (%)<br />
1 0.2 g K2S2O8/2 mL <strong>of</strong> iodine NOM extracted from seaweed<br />
with water/sample pH 6-7/2h stirring<br />
107.2 9.8<br />
2 0.2 g K2S2O8/4 mL <strong>of</strong> iodine HSs extracted from soil with 5%<br />
TMAH/sample pH 10-11/2h stirring<br />
43.6 19.4<br />
3 0.5 g K2S2O8/3 mL <strong>of</strong> iodine NOM extracted from seaweed with<br />
water/sample pH 6-7/2h stirring<br />
100.9 2.5<br />
4 0.5 g K2S2O8/3 mL <strong>of</strong> iodine HSs extracted from soil with 5%<br />
TMAH/sample pH 10-11/2h stirring<br />
47.1 7.6<br />
5 1 g K2S2O8/4 mL mL <strong>of</strong> iodine NOM extracted from seaweed<br />
with water/sample pH 6-7/2h stirring<br />
145.1 5.1<br />
6 1 g K2S2O8/4 mL <strong>of</strong> iodine HSs extracted from soil with 5%<br />
TMAH/sample pH 10-11/2h stirring<br />
49.80<br />
10.0<br />
7 0.5 g K2S2O8/2 mL <strong>of</strong> iodine NOM extracted from seaweed with<br />
water/sample pH 6-7/17h stirring<br />
71.4 13.8<br />
8 1 g K2S2O8/2 mL <strong>of</strong> iodine NOM extracted from seaweed with<br />
water/sample pH 6-7/17h stirring<br />
72.0 11.9<br />
NOM – natural organic matter; HSs – humic substances; TMAH - tetramethylammonium hydroxide;<br />
However, direct precipitation <strong>of</strong> total iodine (without oxidation <strong>of</strong> iodine organic matter) from freshwater as<br />
silver iodine was also per<strong>for</strong>med (Table 10, experiments numbers 11-14). As a result, <strong>the</strong> relative amount <strong>of</strong><br />
total iodine was determined to be 8-15% in <strong>the</strong> filtrate after <strong>the</strong> batch experiment (Table 10, experiments<br />
42