Thesis for the Degree of Doctor of Philosophy - DTU Orbit
Thesis for the Degree of Doctor of Philosophy - DTU Orbit
Thesis for the Degree of Doctor of Philosophy - DTU Orbit
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
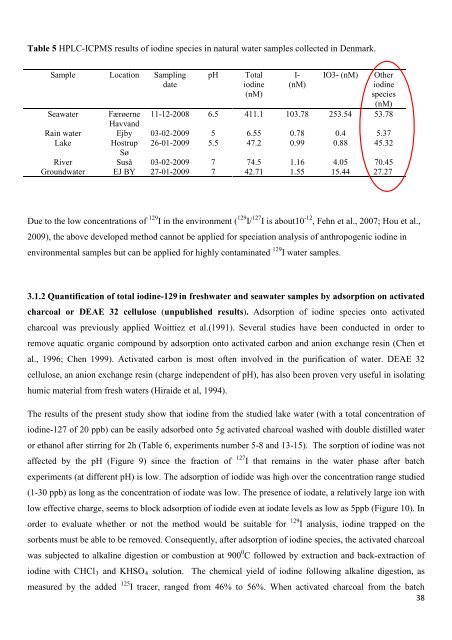
Table 5 HPLC-ICPMS results <strong>of</strong> iodine species in natural water samples collected in Denmark.<br />
Sample Location Sampling<br />
date<br />
pH Total<br />
iodine<br />
(nM)<br />
I-<br />
(nM)<br />
IO3- (nM) O<strong>the</strong>r<br />
iodine<br />
species<br />
(nM)<br />
Seawater Færøerne<br />
Havvand<br />
11-12-2008 6.5 411.1 103.78 253.54 53.78<br />
Rain water Ejby 03-02-2009 5 6.55 0.78 0.4 5.37<br />
Lake Hostrup<br />
Sø<br />
26-01-2009 5.5 47.2 0.99 0.88 45.32<br />
River Suså 03-02-2009 7 74.5 1.16 4.05 70.45<br />
Groundwater EJ BY 27-01-2009 7 42.71 1.55 15.44 27.27<br />
Due to <strong>the</strong> low concentrations <strong>of</strong> 129 I in <strong>the</strong> environment ( 129 I/ 127 I is about10 -12 , Fehn et al., 2007; Hou et al.,<br />
2009), <strong>the</strong> above developed method cannot be applied <strong>for</strong> speciation analysis <strong>of</strong> anthropogenic iodine in<br />
environmental samples but can be applied <strong>for</strong> highly contaminated 129 I water samples.<br />
3.1.2 Quantification <strong>of</strong> total iodine-129 in freshwater and seawater samples by adsorption on activated<br />
charcoal or DEAE 32 cellulose (unpublished results). Adsorption <strong>of</strong> iodine species onto activated<br />
charcoal was previously applied Woittiez et al.(1991). Several studies have been conducted in order to<br />
remove aquatic organic compound by adsorption onto activated carbon and anion exchange resin (Chen et<br />
al., 1996; Chen 1999). Activated carbon is most <strong>of</strong>ten involved in <strong>the</strong> purification <strong>of</strong> water. DEAE 32<br />
cellulose, an anion exchange resin (charge independent <strong>of</strong> pH), has also been proven very useful in isolating<br />
humic material from fresh waters (Hiraide et al, 1994).<br />
The results <strong>of</strong> <strong>the</strong> present study show that iodine from <strong>the</strong> studied lake water (with a total concentration <strong>of</strong><br />
iodine-127 <strong>of</strong> 20 ppb) can be easily adsorbed onto 5g activated charcoal washed with double distilled water<br />
or ethanol after stirring <strong>for</strong> 2h (Table 6, experiments number 5-8 and 13-15). The sorption <strong>of</strong> iodine was not<br />
affected by <strong>the</strong> pH (Figure 9) since <strong>the</strong> fraction <strong>of</strong> 127 I that remains in <strong>the</strong> water phase after batch<br />
experiments (at different pH) is low. The adsorption <strong>of</strong> iodide was high over <strong>the</strong> concentration range studied<br />
(1-30 ppb) as long as <strong>the</strong> concentration <strong>of</strong> iodate was low. The presence <strong>of</strong> iodate, a relatively large ion with<br />
low effective charge, seems to block adsorption <strong>of</strong> iodide even at iodate levels as low as 5ppb (Figure 10). In<br />
order to evaluate whe<strong>the</strong>r or not <strong>the</strong> method would be suitable <strong>for</strong> 129 I analysis, iodine trapped on <strong>the</strong><br />
sorbents must be able to be removed. Consequently, after adsorption <strong>of</strong> iodine species, <strong>the</strong> activated charcoal<br />
was subjected to alkaline digestion or combustion at 900 0 C followed by extraction and back-extraction <strong>of</strong><br />
iodine with CHCl3 and KHSO4 solution. The chemical yield <strong>of</strong> iodine following alkaline digestion, as<br />
measured by <strong>the</strong> added<br />
38<br />
125 I tracer, ranged from 46% to 56%. When activated charcoal from <strong>the</strong> batch