PHYS07200604007 Manas Kumar Dala - Homi Bhabha National ...
PHYS07200604007 Manas Kumar Dala - Homi Bhabha National ...
PHYS07200604007 Manas Kumar Dala - Homi Bhabha National ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Introduction 6<br />
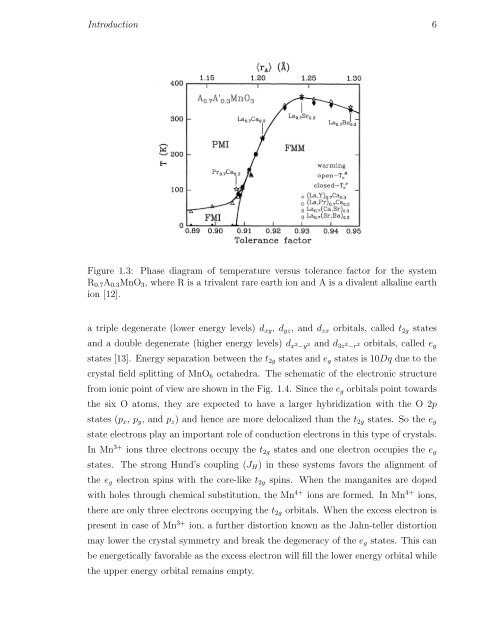
Figure 1.3: Phase diagram of temperature versus tolerance factor for the system<br />
R 0.7 A 0.3 MnO 3 , where R is a trivalent rare earth ion and A is a divalent alkaline earth<br />
ion [12].<br />
a triple degenerate (lower energy levels) d xy , d yz , and d zx orbitals, called t 2g states<br />
and a double degenerate (higher energy levels) d x 2 −y 2 and d 3z 2 −r 2 orbitals, called e g<br />
states [13]. Energy separation between the t 2g states and e g states is 10Dq due to the<br />
crystal field splitting of MnO 6 octahedra. The schematic of the electronic structure<br />
from ionic point of view are shown in the Fig. 1.4. Since the e g orbitals point towards<br />
the six O atoms, they are expected to have a larger hybridization with the O 2p<br />
states (p x , p y , and p z ) and hence are more delocalized than the t 2g states. So the e g<br />
state electrons play an important role of conduction electrons in this type of crystals.<br />
In Mn 3+ ions three electrons occupy the t 2g states and one electron occupies the e g<br />
states. The strong Hund’s coupling (J H ) in these systems favors the alignment of<br />
the e g electron spins with the core-like t 2g spins. When the manganites are doped<br />
with holes through chemical substitution, the Mn 4+ ions are formed. In Mn 4+ ions,<br />
there are only three electrons occupying the t 2g orbitals. When the excess electron is<br />
present in case of Mn 3+ ion, a further distortion known as the Jahn-teller distortion<br />
may lower the crystal symmetry and break the degeneracy of the e g states. This can<br />
be energetically favorable as the excess electron will fill the lower energy orbital while<br />
the upper energy orbital remains empty.