PHYS07200604007 Manas Kumar Dala - Homi Bhabha National ...
PHYS07200604007 Manas Kumar Dala - Homi Bhabha National ...
PHYS07200604007 Manas Kumar Dala - Homi Bhabha National ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Experimental Techniques 24<br />
2.1 Introduction<br />
Spectroscopic methods are some of the most powerful techniques for studying the<br />
electronic structure of solids. This chapter is intended to describe the details of the<br />
different spectroscopic techniques which have been used in this thesis work.<br />
2.2 Photoemission Spectroscopy<br />
2.2.1 Basic principle<br />
The principle of photoemission spectroscopy is based on the principle of photoelectric<br />
effect. The photoelectric effect was first discovered by the German Physicist Heinrich<br />
Hertz [1] in 1887 and latter explained by Albert Einstein [2] in 1905 as a manifestation<br />
of the quantum nature of light.<br />
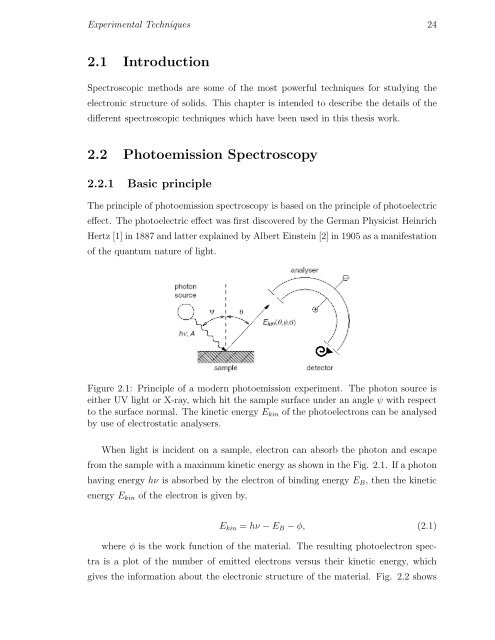
Figure 2.1: Principle of a modern photoemission experiment. The photon source is<br />
either UV light or X-ray, which hit the sample surface under an angle ψ with respect<br />
to the surface normal. The kinetic energy E kin of the photoelectrons can be analysed<br />
by use of electrostatic analysers.<br />
When light is incident on a sample, electron can absorb the photon and escape<br />
from the sample with a maximum kinetic energy as shown in the Fig. 2.1. If a photon<br />
having energy hν is absorbed by the electron of binding energy E B , then the kinetic<br />
energy E kin of the electron is given by,<br />
E kin = hν − E B − φ, (2.1)<br />
where φ is the work function of the material. The resulting photoelectron spectra<br />
is a plot of the number of emitted electrons versus their kinetic energy, which<br />
gives the information about the electronic structure of the material. Fig. 2.2 shows