CHAPTER 3 Tumours of the Stomach - Pathology Outlines
CHAPTER 3 Tumours of the Stomach - Pathology Outlines
CHAPTER 3 Tumours of the Stomach - Pathology Outlines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
A B C<br />
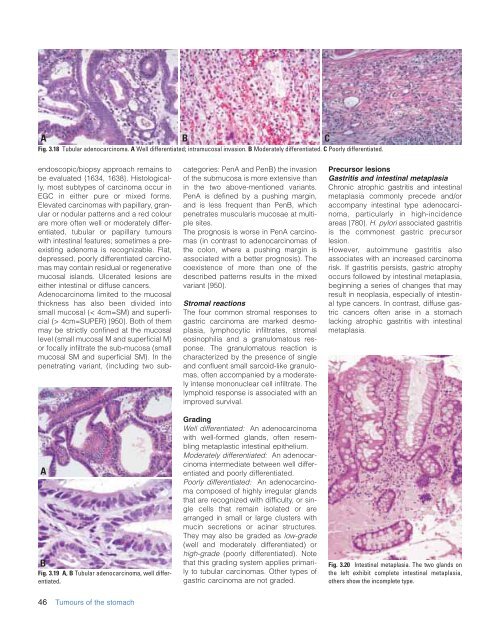
Fig. 3.18 Tubular adenocarcinoma. A Well differentiated; intramucosal invasion. B Moderately differentiated. C Poorly differentiated.<br />
A<br />
B<br />
Fig. 3.19 A, B Tubular adenocarcinoma, well differentiated.<br />
endoscopic/biopsy approach remains to<br />
be evaluated {1634, 1638}. Histologically,<br />
most subtypes <strong>of</strong> carcinoma occur in<br />
EGC in ei<strong>the</strong>r pure or mixed forms.<br />
Elevated carcinomas with papillary, granular<br />
or nodular patterns and a red colour<br />
are more <strong>of</strong>ten well or moderately differentiated,<br />
tubular or papillary tumours<br />
with intestinal features; sometimes a preexisting<br />
adenoma is recognizable. Flat,<br />
depressed, poorly differentiated carcinomas<br />
may contain residual or regenerative<br />
mucosal islands. Ulcerated lesions are<br />
ei<strong>the</strong>r intestinal or diffuse cancers.<br />
Adenocarcinoma limited to <strong>the</strong> mucosal<br />
thickness has also been divided into<br />
small mucosal (< 4cm=SM) and superficial<br />
(> 4cm=SUPER) {950}. Both <strong>of</strong> <strong>the</strong>m<br />
may be strictly confined at <strong>the</strong> mucosal<br />
level (small mucosal M and superficial M)<br />
or focally infiltrate <strong>the</strong> sub-mucosa (small<br />
mucosal SM and superficial SM). In <strong>the</strong><br />
penetrating variant, (including two subcategories:<br />
PenA and PenB) <strong>the</strong> invasion<br />
<strong>of</strong> <strong>the</strong> submucosa is more extensive than<br />
in <strong>the</strong> two above-mentioned variants.<br />
PenA is defined by a pushing margin,<br />
and is less frequent than PenB, which<br />
penetrates muscularis mucosae at multiple<br />
sites.<br />
The prognosis is worse in PenA carcinomas<br />
(in contrast to adenocarcinomas <strong>of</strong><br />
<strong>the</strong> colon, where a pushing margin is<br />
associated with a better prognosis). The<br />
coexistence <strong>of</strong> more than one <strong>of</strong> <strong>the</strong><br />
described patterns results in <strong>the</strong> mixed<br />
variant {950}.<br />
Stromal reactions<br />
The four common stromal responses to<br />
gastric carcinoma are marked desmoplasia,<br />
lymphocytic infiltrates, stromal<br />
eosinophilia and a granulomatous response.<br />
The granulomatous reaction is<br />
characterized by <strong>the</strong> presence <strong>of</strong> single<br />
and confluent small sarcoid-like granulomas,<br />
<strong>of</strong>ten accompanied by a moderately<br />
intense mononuclear cell infiltrate. The<br />
lymphoid response is associated with an<br />
improved survival.<br />
Grading<br />
Well differentiated: An adenocarcinoma<br />
with well-formed glands, <strong>of</strong>ten resembling<br />
metaplastic intestinal epi<strong>the</strong>lium.<br />
Moderately differentiated: An adenocarcinoma<br />
intermediate between well differentiated<br />
and poorly differentiated.<br />
Poorly differentiated: An adenocarcinoma<br />
composed <strong>of</strong> highly irregular glands<br />
that are recognized with difficulty, or single<br />
cells that remain isolated or are<br />
arranged in small or large clusters with<br />
mucin secretions or acinar structures.<br />
They may also be graded as low-grade<br />
(well and moderately differentiated) or<br />
high-grade (poorly differentiated). Note<br />
that this grading system applies primarily<br />
to tubular carcinomas. O<strong>the</strong>r types <strong>of</strong><br />
gastric carcinoma are not graded.<br />
Precursor lesions<br />
Gastritis and intestinal metaplasia<br />
Chronic atrophic gastritis and intestinal<br />
metaplasia commonly precede and/or<br />
accompany intestinal type adenocarcinoma,<br />
particularly in high-incidence<br />
areas {780}. H. pylori associated gastritis<br />
is <strong>the</strong> commonest gastric precursor<br />
lesion.<br />
However, autoimmune gastritis also<br />
associates with an increased carcinoma<br />
risk. If gastritis persists, gastric atrophy<br />
occurs followed by intestinal metaplasia,<br />
beginning a series <strong>of</strong> changes that may<br />
result in neoplasia, especially <strong>of</strong> intestinal<br />
type cancers. In contrast, diffuse gastric<br />
cancers <strong>of</strong>ten arise in a stomach<br />
lacking atrophic gastritis with intestinal<br />
metaplasia.<br />
Fig. 3.20 Intestinal metaplasia. The two glands on<br />
<strong>the</strong> left exhibit complete intestinal metaplasia,<br />
o<strong>the</strong>rs show <strong>the</strong> incomplete type.<br />
46 <strong>Tumours</strong> <strong>of</strong> <strong>the</strong> stomach