Topic 1 - Matter and Energy - Revsworld
Topic 1 - Matter and Energy - Revsworld
Topic 1 - Matter and Energy - Revsworld
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Introduction<br />
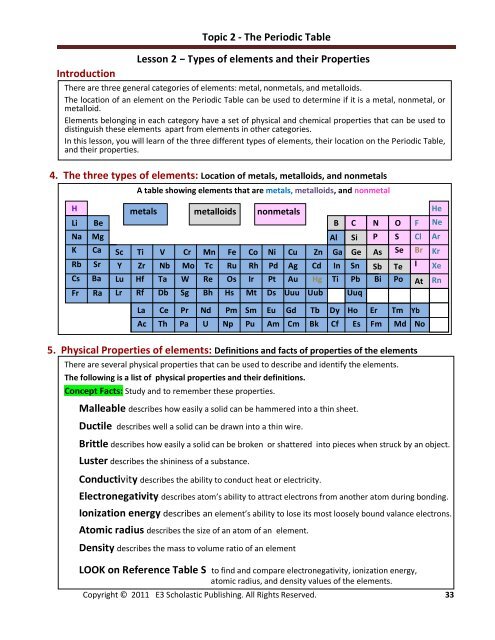
4. The three types of elements: Location of metals, metalloids, <strong>and</strong> nonmetals<br />
.<br />
<strong>Topic</strong> 2 - The Periodic Table<br />
Lesson 2 – Types of elements <strong>and</strong> their Properties<br />
There are three general categories of elements: metal, nonmetals, <strong>and</strong> metalloids.<br />
The location of an element on the Periodic Table can be used to determine if it is a metal, nonmetal, or<br />
metalloid.<br />
Elements belonging in each category have a set of physical <strong>and</strong> chemical properties that can be used to<br />
distinguish these elements apart from elements in other categories.<br />
In this lesson, you will learn of the three different types of elements, their location on the Periodic Table,<br />
<strong>and</strong> their properties.<br />
A table showing elements that are metals, metalloids, <strong>and</strong> nonmetal<br />
H<br />
Li<br />
Na<br />
K<br />
Rb<br />
Cs<br />
Fr<br />
Be<br />
Mg<br />
Ca<br />
Sr<br />
Ba<br />
Ra<br />
metals metalloids nonmetals<br />
B C N O<br />
Al Si P S<br />
Sc Ti V Cr Mn Fe Co Ni Cu Zn Ga Ge As Se<br />
Y Zr Nb Mo Tc Ru Rh Pd Ag Cd In Sn Sb Te<br />
Lu Hf Ta W Re Os Ir Pt Au Hg Ti Pb Bi Po<br />
Lr Rf Db Sg Bh Hs Mt Ds Uuu Uub Uuq<br />
F<br />
Cl<br />
Br<br />
I<br />
At<br />
He<br />
Ne<br />
Ar<br />
Kr<br />
Xe<br />
Rn<br />
La Ce Pr Nd Pm Sm Eu Gd Tb Dy Ho Er Tm Yb<br />
Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No<br />
5. Physical Properties of elements: Definitions <strong>and</strong> facts of properties of the elements<br />
There are several physical properties that can be used to describe <strong>and</strong> identify the elements.<br />
The following is a list of physical properties <strong>and</strong> their definitions.<br />
Concept Facts: Study <strong>and</strong> to remember these properties.<br />
Malleable describes how easily a solid can be hammered into a thin sheet.<br />
Ductile describes well a solid can be drawn into a thin wire.<br />
Brittle describes how easily a solid can be broken or shattered into pieces when struck by an object.<br />
Luster describes the shininess of a substance.<br />
Conductivity describes the ability to conduct heat or electricity.<br />
Electronegativity describes atom’s ability to attract electrons from another atom during bonding.<br />
Ionization energy describes an element’s ability to lose its most loosely bound valance electrons.<br />
Atomic radius describes the size of an atom of an element.<br />
Density describes the mass to volume ratio of an element<br />
LOOK on Reference Table S to find <strong>and</strong> compare electronegativity, ionization energy,<br />
atomic radius, <strong>and</strong> density values of the elements.<br />
Copyright © 2011 E3 Scholastic Publishing. All Rights Reserved. 33