Topic 1 - Matter and Energy - Revsworld
Topic 1 - Matter and Energy - Revsworld
Topic 1 - Matter and Energy - Revsworld
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Lesson 3 – Electrons location <strong>and</strong> arrangement<br />
Introduction<br />
.<br />
<strong>Topic</strong> 3 – The Atomic Structure<br />
According to the wave-mechanical model of atoms, electrons are found in orbital outside the nucleus.<br />
Orbital describes the area (or region) in space outside the nucleus where an electron is likely to be found.<br />
The orbital an electron occupies depends on the energy of the electron. While one electron of an atom<br />
has enough energy to occupy an orbital far from the nucleus, another electron of that same atom may<br />
have just enough energy to occupy a region closer to the nucleus. The result is a formation of energy<br />
levels (or electron shells) around the nucleus of the atom.<br />
The Bohr’s atomic model is often used to show arrangement of electrons in electron shells (energy<br />
levels) of an atom. Each electron shell in Bohr’s atomic model corresponds to the amount of energy the<br />
electrons occupying that shell have.<br />
The arrangement of electrons in atoms is complex. In this lesson, you will learn the basic <strong>and</strong> simplified<br />
arrangement of electrons in electron shells. You will also learn of electron transition (movement) from<br />
one level to another, <strong>and</strong> the production of spectrum of colors.<br />
23. Electron shells: Definitions <strong>and</strong> facts<br />
Electron shells refer to the energy levels in which electrons of an atom occupied.<br />
The following are facts about electron shells of atoms:<br />
Concept Facts: Study to remember the followings about electron shells<br />
. An atom may have one or more electron shells.<br />
. The electron shell (1 st ) closest to the nucleus always contains electrons with least amount of energy<br />
. The electron shell farthest from the nucleus contains electrons with the most amount of energy<br />
. On the Periodic Table, the Period (horizontal row) number indicates the total number of electron<br />
shells in the atoms of elements.<br />
. The electrons configurations found in the box of each element shows the arrangement of electrons in<br />
these electrons shells<br />
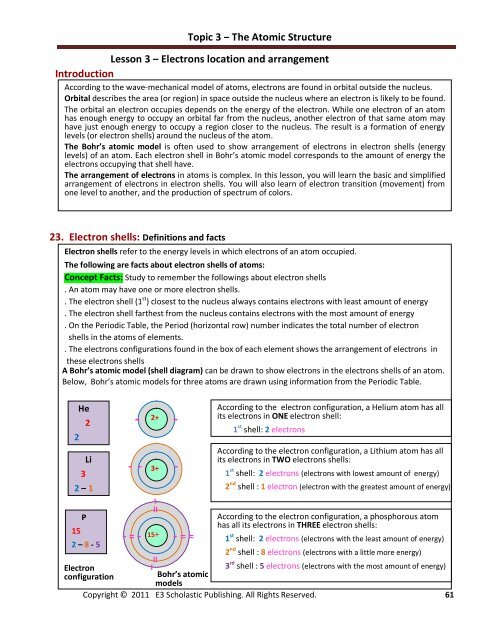
A Bohr’s atomic model (shell diagram) can be drawn to show electrons in the electrons shells of an atom.<br />
Below, Bohr’s atomic models for three atoms are drawn using information from the Periodic Table.<br />
2<br />
He<br />
3<br />
2<br />
Li<br />
2 – 1<br />
15<br />
P<br />
2 – 8 - 5<br />
-<br />
2+<br />
-<br />
- - -<br />
3+<br />
I<br />
II<br />
- = - 15+ - = =<br />
According to the electron configuration, a Helium atom has all<br />
its electrons in ONE electron shell:<br />
1 st shell: 2 electrons<br />
According to the electron configuration, a Lithium atom has all<br />
its electrons in TWO electrons shells:<br />
1 st shell: 2 electrons (electrons with lowest amount of energy)<br />
2 nd shell : 1 electron (electron with the greatest amount of energy)<br />
According to the electron configuration, a phosphorous atom<br />
has all its electrons in THREE electron shells:<br />
1 st shell: 2 electrons (electrons with the least amount of energy)<br />
2 nd shell : 8 electrons (electrons with a little more energy)<br />
Electron<br />
I II 3 rd shell : 5 electrons (electrons with the most amount of energy)<br />
configuration<br />
Bohr’s atomic<br />
models<br />
Copyright © 2011 E3 Scholastic Publishing. All Rights Reserved. 61