Topic 1 - Matter and Energy - Revsworld
Topic 1 - Matter and Energy - Revsworld
Topic 1 - Matter and Energy - Revsworld
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
.<br />
<strong>Topic</strong> 4 – Chemical Bonding<br />
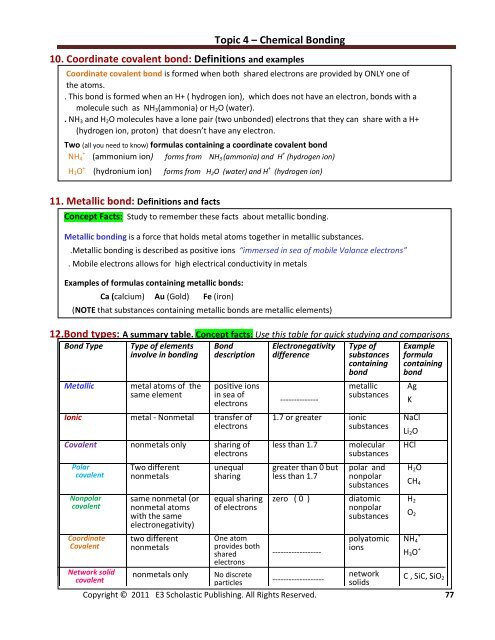
10. Coordinate covalent bond: Definitions <strong>and</strong> examples<br />
Coordinate covalent bond is formed when both shared electrons are provided by ONLY one of<br />
the atoms.<br />
. This bond is formed when an H+ ( hydrogen ion), which does not have an electron, bonds with a<br />
molecule such as NH 3 (ammonia) or H 2 O (water).<br />
. NH 3 <strong>and</strong> H 2 O molecules have a lone pair (two unbonded) electrons that they can share with a H+<br />
(hydrogen ion, proton) that doesn’t have any electron.<br />
Two (all you need to know) formulas containing a coordinate covalent bond<br />
NH 4<br />
+<br />
(ammonium ion) forms from NH 3 (ammonia) <strong>and</strong> H + (hydrogen ion)<br />
H 3 O + (hydronium ion) forms from H 2 O (water) <strong>and</strong> H + (hydrogen ion)<br />
11. Metallic bond: Definitions <strong>and</strong> facts<br />
Concept Facts: Study to remember these facts about metallic bonding.<br />
Metallic bonding is a force that holds metal atoms together in metallic substances.<br />
.Metallic bonding is described as positive ions “immersed in sea of mobile Valance electrons”<br />
. Mobile electrons allows for high electrical conductivity in metals<br />
Examples of formulas containing metallic bonds:<br />
Ca (calcium) Au (Gold) Fe (iron)<br />
(NOTE that substances containing metallic bonds are metallic elements)<br />
12.Bond types: A summary table. Concept facts: Use this table for quick studying <strong>and</strong> comparisons<br />
Bond Type<br />
Metallic<br />
Type of elements<br />
involve in bonding<br />
metal atoms of the<br />
same element<br />
Bond<br />
description<br />
positive ions<br />
in sea of<br />
electrons<br />
Ionic metal - Nonmetal transfer of<br />
electrons<br />
Covalent nonmetals only sharing of<br />
electrons<br />
Polar<br />
covalent<br />
Nonpolar<br />
covalent<br />
Coordinate<br />
Covalent<br />
Network solid<br />
covalent<br />
Two different<br />
nonmetals<br />
same nonmetal (or<br />
nonmetal atoms<br />
with the same<br />
electronegativity)<br />
two different<br />
nonmetals<br />
nonmetals only<br />
unequal<br />
sharing<br />
equal sharing<br />
of electrons<br />
One atom<br />
provides both<br />
shared<br />
electrons<br />
No discrete<br />
particles<br />
Electronegativity<br />
difference<br />
--------------<br />
Type of<br />
substances<br />
containing<br />
bond<br />
metallic<br />
substances<br />
1.7 or greater ionic<br />
substances<br />
less than 1.7<br />
greater than 0 but<br />
less than 1.7<br />
zero ( 0 )<br />
------------------<br />
-------------------<br />
molecular<br />
substances<br />
polar <strong>and</strong><br />
nonpolar<br />
substances<br />
diatomic<br />
nonpolar<br />
substances<br />
polyatomic<br />
ions<br />
network<br />
solids<br />
Example<br />
formula<br />
containing<br />
bond<br />
Copyright © 2011 E3 Scholastic Publishing. All Rights Reserved. 77<br />
Ag<br />
K<br />
NaCl<br />
Li 2 O<br />
HCl<br />
H 2 O<br />
CH 4<br />
H 2<br />
O 2<br />
NH 4<br />
+<br />
H 3 O +<br />
C , SiC, SiO 2