manual of methods for determining micronutrients in fortified foods
manual of methods for determining micronutrients in fortified foods
manual of methods for determining micronutrients in fortified foods
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
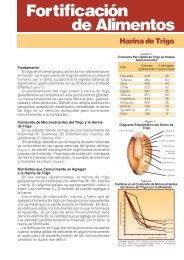
II. Methods <strong>of</strong> analysis <strong>for</strong> iron<br />
Iron is the fourth most abundant element on earth, but iron deficiency <strong>in</strong> humans is one <strong>of</strong> the<br />
most widespread nutritional problems <strong>in</strong> the world, because the human <strong>in</strong>test<strong>in</strong>e has reduced<br />
absorption to most iron compounds. In solid <strong>for</strong>m, metal exists as a malleable metal, which<br />
readily oxidizes <strong>in</strong> moist air.<br />
General Properties<br />
• Symbol Fe<br />
• Mol wt. 55.845 g/mol<br />
• Oxidation states -2 to +6, but +2 and +3 are the most common states.<br />
− Ferrous ion Fe +2<br />
− Ferric ion Fe +3<br />
• Soluble <strong>in</strong> m<strong>in</strong>eral acids Hydrochloric acid, sulfuric acid and nitric acid.<br />
• Ferrous salts oxidize to the ferric <strong>for</strong>m <strong>in</strong> the presence <strong>of</strong> moist air.<br />
• Reacts with chromogenic agents to <strong>for</strong>m complexed colored compounds. The color <strong>of</strong> the complex will vary<br />
depend<strong>in</strong>g on the oxidation state <strong>of</strong> iron. This is helpful when <strong>determ<strong>in</strong><strong>in</strong>g</strong> the presence <strong>of</strong> either ferrous or ferric iron.<br />
A general scheme <strong>of</strong> the steps <strong>in</strong>volved <strong>in</strong> the different techniques <strong>for</strong> measur<strong>in</strong>g iron <strong>in</strong> food is<br />
presented <strong>in</strong> Figure 1. Sample is digested to destroy organic matter and reduce complex molecules to<br />
their elements, us<strong>in</strong>g either wet or dry digestion. A solution from the digested product is prepared with<br />
diluted acid and then the iron content is measured us<strong>in</strong>g Atomic Absorption (AA), Induced-Coupled<br />
Plasma (ICP) or visible spectrophotometry. The visible spectrophotometry technique needs reduction <strong>of</strong><br />
ferric ion to the ferrous <strong>for</strong>m <strong>in</strong> order to use chromogenic <strong>methods</strong> that have larger analytical sensitivity<br />
than those specific <strong>for</strong> ferric iron.<br />
Figure 1. General scheme <strong>for</strong> <strong>determ<strong>in</strong><strong>in</strong>g</strong> iron <strong>in</strong> <strong>foods</strong>.<br />
Several iron compounds are used <strong>for</strong> wheat flour <strong>for</strong>tification, which have different price, bioavailability<br />
and oxidation state. Fortification regulations specify the source <strong>of</strong> iron to be used <strong>in</strong> wheat flour.<br />
However, <strong>methods</strong> <strong>for</strong> <strong>determ<strong>in</strong><strong>in</strong>g</strong> total iron neither dist<strong>in</strong>guish between natural iron and added iron<br />
nor the type <strong>of</strong> iron added (e.g. ferrous, ferric or electrolytic). A method to determ<strong>in</strong>e soluble iron from<br />
ferrous sulfate was developed at Birzeit University and it has been applied rout<strong>in</strong>ely <strong>in</strong> the Central<br />
Public Health Laboratory to determ<strong>in</strong>e whether the source <strong>of</strong> iron used is ferrous sulfate or other. This<br />
<strong>manual</strong> presents three <strong>methods</strong> <strong>for</strong> analyz<strong>in</strong>g iron <strong>in</strong> wheat flour:<br />
• Iron spot test: a qualitative method to determ<strong>in</strong>e the presence <strong>of</strong> iron from <strong>for</strong>tification, regardless<br />
<strong>of</strong> its type, <strong>in</strong> flour.<br />
• Quantitative method <strong>for</strong> <strong>determ<strong>in</strong><strong>in</strong>g</strong> soluble iron from ferrous sulfate.<br />
• Quantitative method <strong>for</strong> <strong>determ<strong>in</strong><strong>in</strong>g</strong> total iron.<br />
- 6 -