Modern Polymer Spect..
Modern Polymer Spect..
Modern Polymer Spect..
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
208 4 T/ihmtional <strong>Spect</strong>roscopy of Ivitact arid Doped Coiijirgnted <strong>Polymer</strong>s<br />
4.2 Materials<br />
The conductivity of iodine-doped polyacetylene first reported by Shirakawa et al.<br />
[I] in 19'7'7 was 30 S cm-'. Since then, the conductivity reported for doped polyacetylene<br />
has kept increasing, the highest conductivity obtained so far for an iodinedoped<br />
stretched polyacetylene film [1?] being > 10' S cm-', a value comparable<br />
with that of copper (6 x 10' S cni-I).<br />
A film of intact polyacetylene usually shows a conductivity lower than lo-'<br />
S cm-' . However, the conductivity increases dramatically when the film is exposed<br />
to oxidizing agents (electron acceptors) such as iodine, AsF5, H'S04, etc. or reducing<br />
agents (electron donors) such as alkali metals. This process is referred to as duping,<br />
by analogy with the doping of inorganic semiconductors. The polymers that are not<br />
doped are referred to as ititact polymers in this review. The main process of doping<br />
is a redox reaction between the polymer chains and acceptors (or donors). Upon<br />
doping, an ionic complex consisting of positively (or negatively) charged polymer<br />
chains and counter ions such as 13-, AsF6-, etc. (or Naf, K+, etc.) is formed.<br />
Counter anions or cations are generated by reduction of acceptors or oxidation of<br />
donors, respectively. The use of an acceptor causes the p-type doping, and that of<br />
a donor the n-type doping. The electrical conductivity can be controlled by the<br />
content of a dopant. A sharp increase in conductivity is observed when the dopant<br />
content is < 1 mole% per C2H2 unit. After this sharp increase, the conductivity becomes<br />
gradually higher with further increase in the dopant content. At low doping<br />
levels, polyacetylene does not exhibit metallic properties, whereas its conductivity is<br />
high. When the dopant content is more than about 13 mole% per C2Hz unit, polyacetylene<br />
shows metallic properties such as a Pauli susceptibility [24, 251, a linear<br />
temperature dependence of the thermoelectric power [26], and a high reflectivity in<br />
the infrared region [27], though the temperature dependence of conductivity is not<br />
like that of a metal. The origin of such properties of heavily doped polyacetylene is<br />
not yet fully understood.<br />
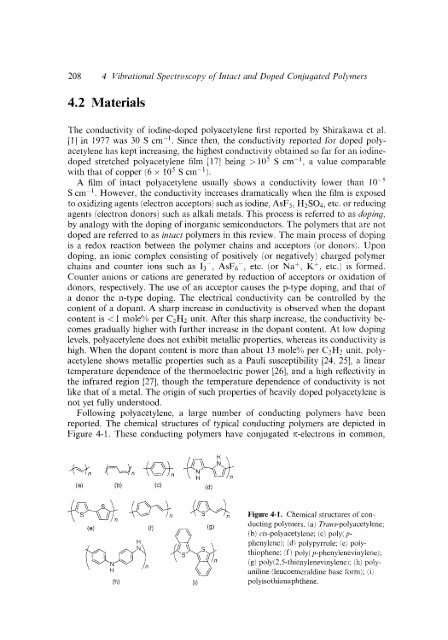
Following polyacetylene, a large number of conducting polymers have been<br />
reported. The chemical structures of typical conducting polymers are depicted in<br />
Figure 4-1. These conducting polymers have conjugated n-electrons in common,<br />
(a) (b) (C) (d)<br />
pq..J-@--q)n<br />
s \ i n<br />
(e)<br />
(f)<br />
p, //<br />
aniline<br />
Figure 4-1. Chemical structures of conducting<br />
polymers. ia) Trurrs-polyacetylene;<br />
(b) cis-polyacetylene; (c) polyj p-<br />
phenylene); (d) polypyrrole; (e) polythiophene:<br />
if 1 poly( p-phenylenevinylene);<br />
(g) poly(2,5-thienylenevinylenei; (h) poly-<br />
(leucoemeraldine base form); (i j<br />
(h) (0 polyisothianaphthene.