teaching - Earth Science Teachers' Association
teaching - Earth Science Teachers' Association
teaching - Earth Science Teachers' Association
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
TEACHING EARTH SCIENCES ● Volume 26 ● Number 3, 2001<br />
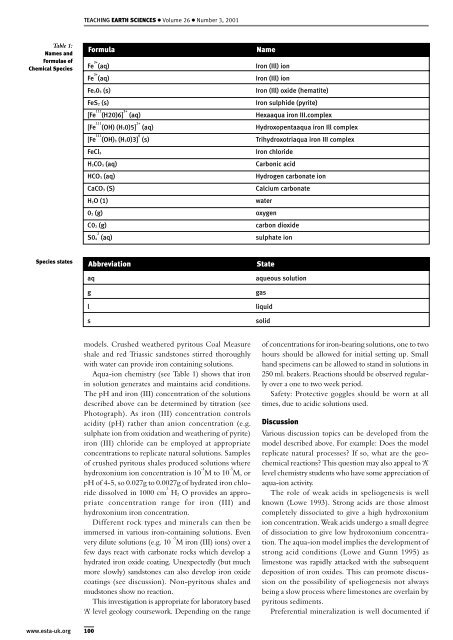
Table 1:<br />
Names and<br />
Formulae of<br />
Chemical Species<br />
Formula<br />
Fe 3+ (aq)<br />
Fe 3+ (aq)<br />
Fe203 (s)<br />
FeS2 (s)<br />
[Fe 111 (H20)6] 3+ (aq)<br />
[Fe 111 (OH) (H20)5] 2+ (aq)<br />
[Fe 111 (OH)3 (H20)3] 0 (s)<br />
FeCl3<br />
H2CO3 (aq)<br />
HCO3 (aq)<br />
CaCO3 (S)<br />
H2O (1)<br />
Iron (Ill) ion<br />
Iron (Ill) ion<br />
Iron (Ill) oxide (hematite)<br />
Iron sulphide (pyrite)<br />
Hexaaqua iron III.complex<br />
Hydroxopentaaqua iron Ill complex<br />
Trihydroxotriaqua iron III complex<br />
Iron chloride<br />
Carbonic acid<br />
Hydrogen carbonate ion<br />
Calcium carbonate<br />
water<br />
02 (g) oxygen<br />
C02 (g)<br />
2<br />
S04 (aq)<br />
Name<br />
carbon dioxide<br />
sulphate ion<br />
Species states<br />
Abbreviation<br />
aq<br />
g<br />
l<br />
s<br />
State<br />
aqueous solution<br />
gas<br />
liquid<br />
solid<br />
models. Crushed weathered pyritous Coal Measure<br />
shale and red Triassic sandstones stirred thoroughly<br />
with water can provide iron containing solutions.<br />
Aqua-ion chemistry (see Table 1) shows that iron<br />
in solution generates and maintains acid conditions.<br />
The pH and iron (III) concentration of the solutions<br />
described above can be determined by titration (see<br />
Photograph). As iron (III) concentration controls<br />
acidity (pH) rather than anion concentration (e.g.<br />
sulphate ion from oxidation and weathering of pyrite)<br />
iron (III) chloride can be employed at appropriate<br />
concentrations to replicate natural solutions. Samples<br />
of crushed pyritous shales produced solutions where<br />
hydroxonium ion concentration is 10 -4 M to 10 -5 M, or<br />
pH of 4-5, so 0.027g to 0.0027g of hydrated iron chloride<br />
dissolved in 1000 cm 3 H 2 O provides an appropriate<br />
concentration range for iron (III) and<br />
hydroxonium iron concentration.<br />
Different rock types and minerals can then be<br />
immersed in various iron-containing solutions. Even<br />
very dilute solutions (e.g. 10 -5 M iron (III) ions) over a<br />
few days react with carbonate rocks which develop a<br />
hydrated iron oxide coating. Unexpectedly (but much<br />
more slowly) sandstones can also develop iron oxide<br />
coatings (see discussion). Non-pyritous shales and<br />
mudstones show no reaction.<br />
This investigation is appropriate for laboratory based<br />
‘A’ level geology coursework. Depending on the range<br />
of concentrations for iron-bearing solutions, one to two<br />
hours should be allowed for initial setting up. Small<br />
hand specimens can be allowed to stand in solutions in<br />
250 ml. beakers. Reactions should be observed regularly<br />
over a one to two week period.<br />
Safety: Protective goggles should be worn at all<br />
times, due to acidic solutions used.<br />
Discussion<br />
Various discussion topics can be developed from the<br />
model described above. For example: Does the model<br />
replicate natural processes? If so, what are the geochemical<br />
reactions? This question may also appeal to ‘A’<br />
level chemistry students who have some appreciation of<br />
aqua-ion activity.<br />
The role of weak acids in speliogenesis is well<br />
known (Lowe 1993). Strong acids are those almost<br />
completely dissociated to give a high hydroxonium<br />
ion concentration. Weak acids undergo a small degree<br />
of dissociation to give low hydroxonium concentration.<br />
The aqua-ion model implies the development of<br />
strong acid conditions (Lowe and Gunn 1995) as<br />
limestone was rapidly attacked with the subsequent<br />
deposition of iron oxides. This can promote discussion<br />
on the possibility of speliogenesis not always<br />
being a slow process where limestones are overlain by<br />
pyritous sediments.<br />
Preferential mineralization is well documented if<br />
www.esta-uk.org<br />
100