annals 1-2.qxd - Centrum Zdrowia Dziecka
annals 1-2.qxd - Centrum Zdrowia Dziecka
annals 1-2.qxd - Centrum Zdrowia Dziecka
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
8<br />
specific gliadin peptides that have been implicated in CD.<br />
HLA-DQ molecules present on the surface of antigen presenting<br />
cells (macrophages, dendritic cells), i.e. cells, which<br />
catch the gliadin peptides and present them in association<br />
with specific HLA-DQ receptors to T lymphocytes [43].<br />
Gluten contains peptides presenting extremely high<br />
affinity to DQ2 and DQ8 receptors [51]. These toxic peptides<br />
are characterized by high glutamine content, i.e. the amino<br />
acid which is a substrate for the enzyme – tissue transglutaminase<br />
(tTG). tTG catalyzes deamination of glutamine into<br />
glutamine acid. This reaction increases about 100-times<br />
the affinity of toxic peptides, and is essential for significant<br />
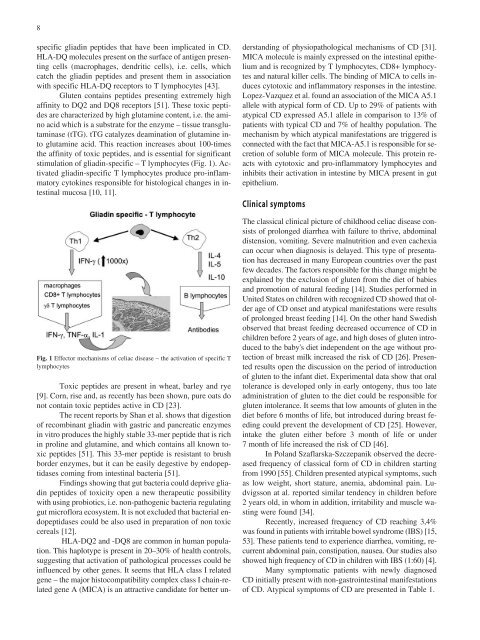
stimulation of gliadin-specific – T lymphocytes (Fig. 1). Activated<br />
gliadin-specific T lymphocytes produce pro-inflammatory<br />
cytokines responsible for histological changes in intestinal<br />
mucosa [10, 11].<br />
Fig. 1 Effector mechanisms of celiac disease – the activation of specific T<br />
lymphocytes<br />
Toxic peptides are present in wheat, barley and rye<br />
[9]. Corn, rise and, as recently has been shown, pure oats do<br />
not contain toxic peptides active in CD [23].<br />
The recent reports by Shan et al. shows that digestion<br />
of recombinant gliadin with gastric and pancreatic enzymes<br />
in vitro produces the highly stable 33-mer peptide that is rich<br />
in proline and glutamine, and which contains all known toxic<br />
peptides [51]. This 33-mer peptide is resistant to brush<br />
border enzymes, but it can be easily degestive by endopeptidases<br />
coming from intestinal bacteria [51].<br />
Findings showing that gut bacteria could deprive gliadin<br />
peptides of toxicity open a new therapeutic possibility<br />
with using probiotics, i.e. non-pathogenic bacteria regulating<br />
gut microflora ecosystem. It is not excluded that bacterial endopeptidases<br />
could be also used in preparation of non toxic<br />
cereals [12].<br />
HLA-DQ2 and -DQ8 are common in human population.<br />
This haplotype is present in 20–30% of health controls,<br />
suggesting that activation of pathological processes could be<br />
influenced by other genes. It seems that HLA class I related<br />
gene – the major histocompatibility complex class I chain-related<br />
gene A (MICA) is an attractive candidate for better un-<br />
derstanding of physiopathological mechanisms of CD [31].<br />
MICA molecule is mainly expressed on the intestinal epithelium<br />
and is recognized by T lymphocytes, CD8+ lymphocytes<br />
and natural killer cells. The binding of MICA to cells induces<br />
cytotoxic and inflammatory responses in the intestine.<br />
Lopez-Vazquez et al. found an association of the MICA A5.1<br />
allele with atypical form of CD. Up to 29% of patients with<br />
atypical CD expressed A5.1 allele in comparison to 13% of<br />
patients with typical CD and 7% of healthy population. The<br />
mechanism by which atypical manifestations are triggered is<br />
connected with the fact that MICA-A5.1 is responsible for secretion<br />
of soluble form of MICA molecule. This protein reacts<br />
with cytotoxic and pro-inflammatory lymphocytes and<br />
inhibits their activation in intestine by MICA present in gut<br />
epithelium.<br />
Clinical symptoms<br />
The classical clinical picture of childhood celiac disease consists<br />
of prolonged diarrhea with failure to thrive, abdominal<br />
distension, vomiting. Severe malnutrition and even cachexia<br />
can occur when diagnosis is delayed. This type of presentation<br />
has decreased in many European countries over the past<br />
few decades. The factors responsible for this change might be<br />
explained by the exclusion of gluten from the diet of babies<br />
and promotion of natural feeding [14]. Studies performed in<br />
United States on children with recognized CD showed that older<br />
age of CD onset and atypical manifestations were results<br />
of prolonged breast feeding [14]. On the other hand Swedish<br />
observed that breast feeding decreased occurrence of CD in<br />
children before 2 years of age, and high doses of gluten introduced<br />
to the baby's diet independent on the age without protection<br />
of breast milk increased the risk of CD [26]. Presented<br />
results open the discussion on the period of introduction<br />
of gluten to the infant diet. Experimental data show that oral<br />
tolerance is developed only in early ontogeny, thus too late<br />
administration of gluten to the diet could be responsible for<br />
gluten intolerance. It seems that low amounts of gluten in the<br />
diet before 6 months of life, but introduced during breast feeding<br />
could prevent the development of CD [25]. However,<br />
intake the gluten either before 3 month of life or under<br />
7 month of life increased the risk of CD [46].<br />
In Poland Szaflarska-Szczepanik observed the decreased<br />
frequency of classical form of CD in children starting<br />
from 1990 [55]. Children presented atypical symptoms, such<br />
as low weight, short stature, anemia, abdominal pain. Ludvigsson<br />
at al. reported similar tendency in children before<br />
2 years old, in whom in addition, irritability and muscle wasting<br />
were found [34].<br />
Recently, increased frequency of CD reaching 3,4%<br />
was found in patients with irritable bowel syndrome (IBS) [15,<br />
53]. These patients tend to experience diarrhea, vomiting, recurrent<br />
abdominal pain, constipation, nausea. Our studies also<br />
showed high frequency of CD in children with IBS (1:60) [4].<br />
Many symptomatic patients with newly diagnosed<br />
CD initially present with non-gastrointestinal manifestations<br />
of CD. Atypical symptoms of CD are presented in Table 1.