EGAS41 - Swansea University
EGAS41 - Swansea University
EGAS41 - Swansea University
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
41 st EGAS CP 69 Gdańsk 2009<br />
Fragmentation of multiply charged clusters<br />
M. Nakamura ∗<br />
College of Science and Technology, Nihon <strong>University</strong>, Japan<br />
∗ Corresponding author: mooming@phys.ge.cst.nihon-u.ac.jp<br />
A multiply charged cluster is stable if its size is larger than the appearance size. Otherwise,<br />
the cluster fragments automatically. The stability and fragmentation of van der Waals<br />
clusters have been discussed within the framework of the liquid drop model (LDM) [1].<br />
Recently, the author has studied the role of geometrical shell effect to the appearance size<br />
of multiply charged clusters [2,3]. Here we discuss influences of the shell effect on the decay<br />
of multiply charged clusters. The shell energy is estimated from the calculation of the<br />
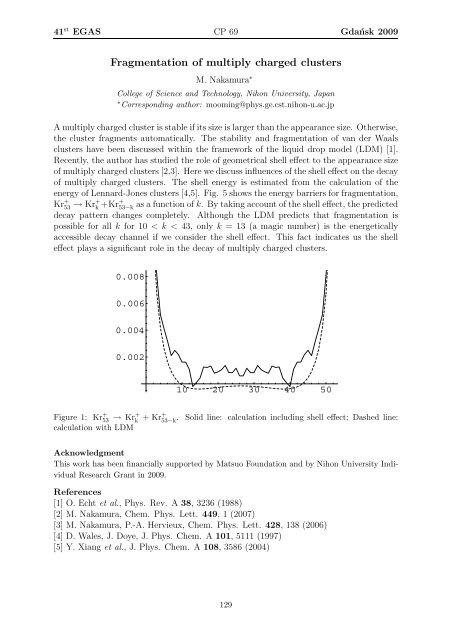
energy of Lennard-Jones clusters [4,5]. Fig. 5 shows the energy barriers for fragmentation,<br />
Kr + 53 → Kr+ k +Kr+ 53−k as a function of k. By taking account of the shell effect, the predicted<br />
decay pattern changes completely. Although the LDM predicts that fragmentation is<br />
possible for all k for 10 < k < 43, only k = 13 (a magic number) is the energetically<br />
accessible decay channel if we consider the shell effect. This fact indicates us the shell<br />
effect plays a significant role in the decay of multiply charged clusters.<br />
0.008<br />
0.006<br />
0.004<br />
0.002<br />
10 20 30 40 50<br />
Figure 1: Kr + 53 → Kr+ k + Kr+ 53−k<br />
. Solid line: calculation including shell effect; Dashed line:<br />
calculation with LDM<br />
Acknowledgment<br />
This work has been financially supported by Matsuo Foundation and by Nihon <strong>University</strong> Individual<br />
Research Grant in 2009.<br />
References<br />
[1] O. Echt et al., Phys. Rev. A 38, 3236 (1988)<br />
[2] M. Nakamura, Chem. Phys. Lett. 449, 1 (2007)<br />
[3] M. Nakamura, P.-A. Hervieux, Chem. Phys. Lett. 428, 138 (2006)<br />
[4] D. Wales, J. Doye, J. Phys. Chem. A 101, 5111 (1997)<br />
[5] Y. Xiang et al., J. Phys. Chem. A 108, 3586 (2004)<br />
129