1. Basic Concepts in Reliability Design - nl3prc
1. Basic Concepts in Reliability Design - nl3prc
1. Basic Concepts in Reliability Design - nl3prc
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
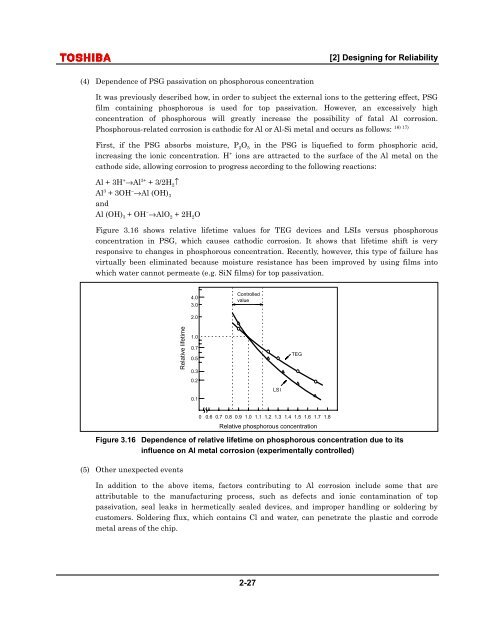
[2] <strong>Design</strong><strong>in</strong>g for <strong>Reliability</strong>(4) Dependence of PSG passivation on phosphorous concentrationIt was previously described how, <strong>in</strong> order to subject the external ions to the getter<strong>in</strong>g effect, PSGfilm conta<strong>in</strong><strong>in</strong>g phosphorous is used for top passivation. However, an excessively highconcentration of phosphorous will greatly <strong>in</strong>crease the possibility of fatal Al corrosion.16) 17)Phosphorous-related corrosion is cathodic for Al or Al-Si metal and occurs as follows:First, if the PSG absorbs moisture, P 2 O 5 <strong>in</strong> the PSG is liquefied to form phosphoric acid,<strong>in</strong>creas<strong>in</strong>g the ionic concentration. H + ions are attracted to the surface of the Al metal on thecathode side, allow<strong>in</strong>g corrosion to progress accord<strong>in</strong>g to the follow<strong>in</strong>g reactions:Al + 3H + →Al 3+ + 3/2H 2 ↑Al 3 + 3OH − →Al (OH) 3andAl (OH) 3 + OH − →AlO 2 + 2H 2 OFigure 3.16 shows relative lifetime values for TEG devices and LSIs versus phosphorousconcentration <strong>in</strong> PSG, which causes cathodic corrosion. It shows that lifetime shift is veryresponsive to changes <strong>in</strong> phosphorous concentration. Recently, however, this type of failure hasvirtually been elim<strong>in</strong>ated because moisture resistance has been improved by us<strong>in</strong>g films <strong>in</strong>towhich water cannot permeate (e.g. SiN films) for top passivation.4.03.0Controlledvalue2.0Relative lifetime<strong>1.</strong>00.70.50.30.20.1LSITEG0 0.6 0.7 0.8 0.9 <strong>1.</strong>0 <strong>1.</strong>1 <strong>1.</strong>2 <strong>1.</strong>3 <strong>1.</strong>4 <strong>1.</strong>5 <strong>1.</strong>6 <strong>1.</strong>7 <strong>1.</strong>8Relative phosphorous concentrationFigure 3.16 Dependence of relative lifetime on phosphorous concentration due to its<strong>in</strong>fluence on Al metal corrosion (experimentally controlled)(5) Other unexpected eventsIn addition to the above items, factors contribut<strong>in</strong>g to Al corrosion <strong>in</strong>clude some that areattributable to the manufactur<strong>in</strong>g process, such as defects and ionic contam<strong>in</strong>ation of toppassivation, seal leaks <strong>in</strong> hermetically sealed devices, and improper handl<strong>in</strong>g or solder<strong>in</strong>g bycustomers. Solder<strong>in</strong>g flux, which conta<strong>in</strong>s Cl and water, can penetrate the plastic and corrodemetal areas of the chip.2-27