Diffusion Processes with Hidden States from ... - FU Berlin, FB MI
Diffusion Processes with Hidden States from ... - FU Berlin, FB MI
Diffusion Processes with Hidden States from ... - FU Berlin, FB MI
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
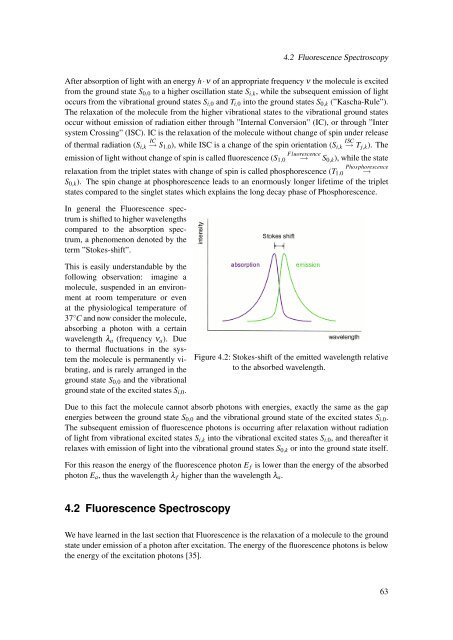
4.2 Fluorescence SpectroscopyAfter absorption of light <strong>with</strong> an energy h·ν of an appropriate frequency ν the molecule is excited<strong>from</strong> the ground state S 0,0 to a higher oscillation state S i,k , while the subsequent emission of lightoccurs <strong>from</strong> the vibrational ground states S i,0 and T i,0 into the ground states S 0,k (”Kascha-Rule”).The relaxation of the molecule <strong>from</strong> the higher vibrational states to the vibrational ground statesoccur <strong>with</strong>out emission of radiation either through ”Internal Conversion” (IC), or through ”Intersystem Crossing” (ISC). IC is the relaxation of the molecule <strong>with</strong>out change of spin under releaseIC ISCof thermal radiation (S i,k → S1,0 ), while ISC is a change of the spin orientation (S i,k → T j,k ). TheFluorescenceemission of light <strong>with</strong>out change of spin is called fluorescence (S 1,0 → S 0,k ), while the statePhosphorescencerelaxation <strong>from</strong> the triplet states <strong>with</strong> change of spin is called phosphorescence (T 1,0 →S 0,k ). The spin change at phosphorescence leads to an enormously longer lifetime of the tripletstates compared to the singlet states which explains the long decay phase of Phosphorescence.In general the Fluorescence spectrumis shifted to higher wavelengthscompared to the absorption spectrum,a phenomenon denoted by theterm ”Stokes-shift”.This is easily understandable by thefollowing observation: imagine amolecule, suspended in an environmentat room temperature or evenat the physiological temperature of37 ◦ C and now consider the molecule,absorbing a photon <strong>with</strong> a certainwavelength λ a (frequency ν a ). Dueto thermal fluctuations in the systemthe molecule is permanently vibrating,and is rarely arranged in theground state S 0,0 and the vibrationalground state of the excited states S i,0 .Figure 4.2: Stokes-shift of the emitted wavelength relativeto the absorbed wavelength.Due to this fact the molecule cannot absorb photons <strong>with</strong> energies, exactly the same as the gapenergies between the ground state S 0,0 and the vibrational ground state of the excited states S i,0 .The subsequent emission of fluorescence photons is occurring after relaxation <strong>with</strong>out radiationof light <strong>from</strong> vibrational excited states S i,k into the vibrational excited states S i,0 , and thereafter itrelaxes <strong>with</strong> emission of light into the vibrational ground states S 0,k or into the ground state itself.For this reason the energy of the fluorescence photon E f is lower than the energy of the absorbedphoton E a , thus the wavelength λ f higher than the wavelength λ a .4.2 Fluorescence SpectroscopyWe have learned in the last section that Fluorescence is the relaxation of a molecule to the groundstate under emission of a photon after excitation. The energy of the fluorescence photons is belowthe energy of the excitation photons [35].63