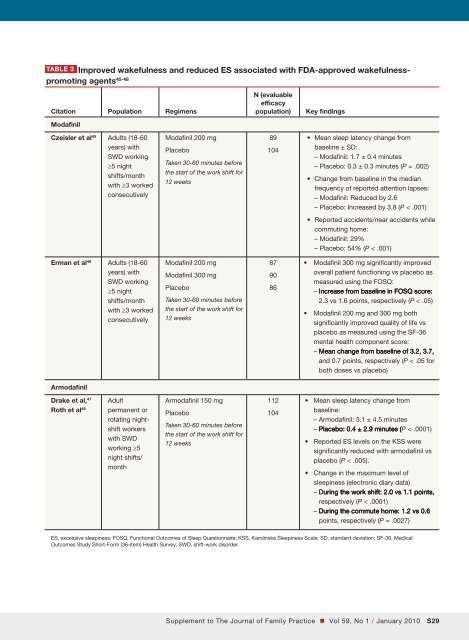
Shift-work disorderfor a motor vehicle accident during the <strong>com</strong>mute home.Practical steps are summarized in TABLE 2.Attention to dietOne study has suggested that attention to dietary <strong>com</strong>positionmay have an impact on alertness and performanceamong individuals working night shifts in ahospital setting. 44 The study suggested that a diet witha carbohydrate-to-protein ratio of around 3:1 is optimalin terms of benefits for both mood and psychometricperformance.Pharmacologic interventionsWakefulness-promoting agentsThe wakefulness-promoting agents modafinil and armodafinil(the R-enantiomer of modafinil) are currentlythe only agents specifically approved by the FDA forthe treatment of ES associated with SWD. Approval ofmodafinil for this indication was based on the resultsof 2 controlled clinical trials (TABLE 3). 45,46 Modafinil significantlyimproved wakefulness, as measured using patient-reporteddiary data and changes on the MultipleSleep Latency Test (P < .001 and P = .002, respectively) inthose who had ES as a consequence of SWD. 45 Attentionwas also significantly improved in the modafinil group<strong>com</strong>pared with placebo (P < .001), and significantlyfewer participants treated with modafinil reported accidentsor near misses during the <strong>com</strong>mute home thandid those who received placebo (P < .001). 45 Additionally,modafinil significantly improved self-reports of functioning(in terms of productivity and vigilance; P < .05)and quality of life (P < .05) in individuals with SWD. 46In these 2 studies, headache was the most <strong>com</strong>monlyreported adverse event, and nausea was thenext most prominent adverse effect with modafinil. Inthe study by Czeisler and colleagues 45 more modafiniltreatedpatients experienced insomnia <strong>com</strong>pared withthe placebo group (6% vs 0%, respectively; P < .01).Armodafinil has been shown to improve wakefulnessin individuals with ES associated with SWD in acontrolled clinical trial (TABLE 3). 47,48 This study showedarmodafinil to be significantly better than placebo atimproving wakefulness, reflected by a significantly prolongedsleep latency throughout the night among nightshiftworkers with SWD (P < .0001). Compared withplacebo, treatment with a single dose of armodafinil150 mg, 30 to 60 minutes before the start of the shift, significantlyreduced ES at work (P < .0001) and during the<strong>com</strong>mute home (P = .0027) and did not adversely affectdaytime sleep. 47,48 As observed for modafinil, headacheand nausea were the most <strong>com</strong>mon treatment-emergentadverse events in patients with SWD who took partin these 2 studies.To date, no studies have been performed that directly<strong>com</strong>pare the efficacy of armodafinil and modafinil;however, the 2 wakefulness-promoting agents do havedifferent pharmacokinetic profiles. 49,50 Compared withmodafinil, armodafinil takes longer to reach its peakplasma concentration and is present at higher concentrationsfor a longer period after administration, resultingin its wakefulness-promoting effects lasting throughoutthe day. 49,50 The longer duration of armodafinil’s effectsand its potential for once-daily dosing make it an appropriateand convenient choice for patients with SWD.StimulantsStimulants, such as methamphetamine, have beenshown to enhance wakefulness in individuals undergoingsimulated night-shift work. 51,52 However, amphetaminescan induce rebound insomnia and this, <strong>com</strong>binedwith their adverse cardiovascular effects and theirabuse potential, makes them less than ideal options foran often chronic condition such as SWD. 53 Methamphetaminehas not been evaluated as an interventionfor individuals with a diagnosis of SWD and, althoughit is effective at improving performance and mood duringone or more night shifts after single doses, its usefulnessin managing SWD on numerous sequential nightsis questionable.A number of studies among individuals undergoingsimulated night-shift work suggest that caffeine may beuseful to promote wakefulness during the work period,although there may be some residual effects on daytimesleep depending on the caffeine drink selected. 54-56 Onestudy suggested that low-dose repeated caffeine administrationmay improve performance at the expenseof increasing subjective ES during periods of extendedwakefulness. 57 As discussed above, caffeine in <strong>com</strong>binationwith other wakefulness-promoting strategies,including scheduled napping and bright light therapy,has proved to be a promising intervention under simulatedshift-work conditions. 22,23 However, the appropriatedose and timing of caffeine intake to optimizeperformance and mood during a night shift have not yetbeen determined. Higher caffeine doses may induce astate of hyperstimulation and can even be toxic. 58 Moreover,habitual caffeine intake can lead to the developmentof tolerance to its effects, 59 abrogating the efficacyof caffeine intake in the long-term management of anoften chronic condition such as SWD. To date, regularS28 January 2010 / Vol 59, No 1 • Supplement to The Journal of Family Practice
Table 3 Improved wakefulness and reduced ES associated with FDA-approved wakefulness-promoting agents 45-48Citation Population RegimensModafinilN (evaluableefficacypopulation)Key findingsCzeisler et al 45 Adults (18-60years) withSWD working≥5 nightshifts/monthwith ≥3 workedconsecutivelyModafinil 200 mgPlaceboTaken 30-60 minutes beforethe start of the work shift for12 weeks89104• Mean sleep latency change frombaseline ± SD:– Modafinil: 1.7 ± 0.4 minutes– Placebo: 0.3 ± 0.3 minutes (P = .002)• Change from baseline in the medianfrequency of reported attention lapses:– Modafinil: Reduced by 2.6– Placebo: Increased by 3.8 (P < .001)• Reported accidents/near accidents while<strong>com</strong>muting home:– Modafinil: 29%– Placebo: 54% (P < .001)Erman et al 46 Adults (18-60years) withSWD working≥5 nightshifts/monthwith ≥3 workedconsecutivelyModafinil 200 mgModafinil 300 mgPlaceboTaken 30-60 minutes beforethe start of the work shift for12 weeks879086• Modafinil 300 mg significantly improvedoverall patient functioning vs placebo asmeasured using the FOSQ:– Increase from baseline in FoSQ FOSQ score:2.3 vs 1.6 points, respectively (P < .05)• Modafinil 200 mg and 300 mg bothsignificantly improved quality of life vsplacebo as measured using the SF-36mental health <strong>com</strong>ponent score:– mean Mean change from baseline of 3.2, 3.7,and 0.7 points, respectively (P < .05 forboth doses vs placebo)ArmodafinilDrake et al, 47Roth et al 48Adultpermanent orrotating nightshiftworkerswith SWDworking ≥5night shifts/monthArmodafinil 150 mgPlaceboTaken 30-60 minutes beforethe start of the work shift for12 weeks112104• Mean sleep latency change frombaseline:– Armodafinil: 3.1 ± 4.5 minutes– placebo: Placebo: 0.4 ± 2.9 minutes (P < .0001)• Reported ES levels on the KSS weresignificantly reduced with armodafinil vsplacebo (P < .005).• Change in the maximum level ofsleepiness (electronic diary data)– During the work shift: 2.0 vs 1.1 points,respectively (P < .0001)– During the <strong>com</strong>mute home: 1.2 vs 0.6points, respectively (P = .0027)ES, excessive sleepiness; FOSQ, Functional Out<strong>com</strong>es of Sleep Questionnaire; KSS, Karolinska Sleepiness Scale; SD, standard deviation; SF-36, MedicalOut<strong>com</strong>es Study Short-Form (36-item) Health Survey; SWD, shift-work disorder.Supplement to The Journal of Family Practice • Vol 59, No 1 / January 2010 S29
- Page 1 and 2:
Practice Management Tips ForSHIFT W
- Page 3 and 4:
Patient QuestionnaireDo you often f
- Page 5 and 6:
Sleep/Wake LogIn bedOut of bedLight
- Page 7 and 8:
PHQ-9 QUICK DEPRESSION ASSESSMENTFo
- Page 9 and 10:
Insomnia Severity IndexPlease answe
- Page 11 and 12:
Take-Away PointsSHIFT WORK DISORDER
- Page 13 and 14:
SHIFT WORKDISORDERBright Light Ther
- Page 40 and 41: PrimarycareScreeningfor depressioni
- Page 42 and 43: PrimarycareThescreening questionnai
- Page 44 and 45: Shift-work disorderContents and Fac
- Page 46 and 47: Shift-work disorderThe diagnosis of
- Page 48 and 49: Shift-work disorderas heightened le
- Page 50 and 51: Shift-work disorderFigure 1 Risk ra
- Page 52 and 53: Shift-work disorderare not function
- Page 54 and 55: The characterization andpathology o
- Page 56 and 57: Shift-work disorderFigure 2 Sleep/w
- Page 58 and 59: Shift-work disorderFigure 3 Blood p
- Page 60 and 61: Recognition of shift-workdisorder i
- Page 62 and 63: Shift-work disorderThe timing of sh
- Page 64 and 65: Shift-work disorderthe other potent
- Page 66 and 67: Managing the patient withshift-work
- Page 68 and 69: Shift-work disorderFigure 3 Optimal
- Page 72 and 73: Shift-work disordermoderate caffein
- Page 74 and 75: Supplement toAvailable at jfponline
- Page 76 and 77: Armodafinil for Treatment of Excess
- Page 78 and 79: Armodafinil for Treatment of Excess
- Page 80 and 81: Armodafinil for Treatment of Excess
- Page 82 and 83: Armodafinil for Treatment of Excess
- Page 84 and 85: Armodafinil for Treatment of Excess
- Page 86 and 87: Armodafinil for Treatment of Excess
- Page 88 and 89: Armodafinil for Treatment of Excess
- Page 90 and 91: Armodafinil for Treatment of Excess
- Page 92 and 93: Armodafinil for Treatment of Excess
- Page 94 and 95: Armodafinil for Treatment of Excess
- Page 96 and 97: Armodafinil for Treatment of Excess
- Page 98 and 99: The Epidemiology and Diagnosis of I
- Page 100 and 101: The Epidemiology and Diagnosis of I
- Page 102 and 103: The Epidemiology and Diagnosis of I
- Page 120 and 121:
CIRCADIAN RHYTHM SLEEP DISORDERSPra
- Page 122 and 123:
Table 2— AASM Levels of Recommend
- Page 124 and 125:
3.2.1.1 Both the Morningness-Evenin
- Page 126 and 127:
Five studies used one of the newer
- Page 128 and 129:
as an indicator of phase in sighted
- Page 130 and 131:
4.4 Advanced Sleep Phase DisorderBe
- Page 132 and 133:
45. Walsh, JK, Randazzo, AC, Stone,
- Page 134:
123. Van Someren, EJ, Kessler, A, M
- Page 142 and 143:
Table 1—Subject Demographicsn M:F
- Page 144 and 145:
Scale. 28 The simple reaction time
- Page 146 and 147:
Median RT (msec)1600A14001200100080
- Page 148 and 149:
10Mentally AExhaustedSharpScore8642
- Page 150 and 151:
Current Treatment Options in Neurol
- Page 152 and 153:
398 Sleep Disordersand sleep loss,
- Page 154 and 155:
400 Sleep DisordersTable 1. Treatme
- Page 156 and 157:
402 Sleep DisordersStandard dosageC
- Page 158 and 159:
404 Sleep DisordersStandard procedu
- Page 160 and 161:
406 Sleep DisordersCaffeineMelatoni
- Page 162 and 163:
408 Sleep DisordersWake-promoting a
- Page 164 and 165:
410 Sleep Disordersnight shift: ada