analysis of water injection into high-temperature mixture of ...
analysis of water injection into high-temperature mixture of ...
analysis of water injection into high-temperature mixture of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
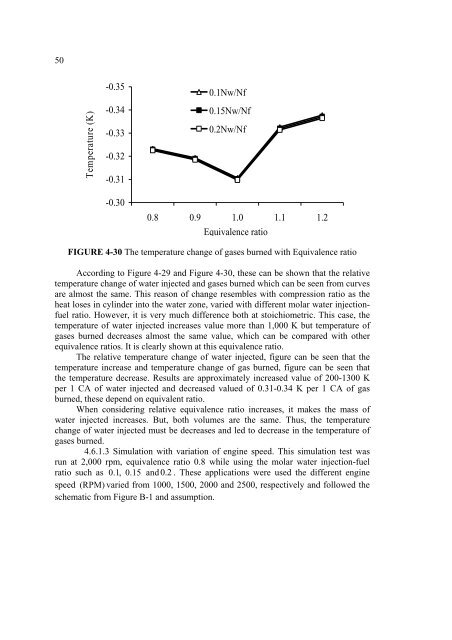
50Temperature (K)-0.35-0.34-0.33-0.32-0.31-0.300.1Nw/Nf0.15Nw/Nf0.2Nw/Nf0.8 0.9 1.0 1.1 1.2Equivalence ratioFIGURE 4-30 The <strong>temperature</strong> change <strong>of</strong> gases burned with Equivalence ratioAccording to Figure 4-29 and Figure 4-30, these can be shown that the relative<strong>temperature</strong> change <strong>of</strong> <strong>water</strong> injected and gases burned which can be seen from curvesare almost the same. This reason <strong>of</strong> change resembles with compression ratio as theheat loses in cylinder <strong>into</strong> the <strong>water</strong> zone, varied with different molar <strong>water</strong> <strong>injection</strong>fuelratio. However, it is very much difference both at stoichiometric. This case, the<strong>temperature</strong> <strong>of</strong> <strong>water</strong> injected increases value more than 1,000 K but <strong>temperature</strong> <strong>of</strong>gases burned decreases almost the same value, which can be compared with otherequivalence ratios. It is clearly shown at this equivalence ratio.The relative <strong>temperature</strong> change <strong>of</strong> <strong>water</strong> injected, figure can be seen that the<strong>temperature</strong> increase and <strong>temperature</strong> change <strong>of</strong> gas burned, figure can be seen thatthe <strong>temperature</strong> decrease. Results are approximately increased value <strong>of</strong> 200-1300 Kper 1 CA <strong>of</strong> <strong>water</strong> injected and decreased valued <strong>of</strong> 0.31-0.34 K per 1 CA <strong>of</strong> gasburned, these depend on equivalent ratio.When considering relative equivalence ratio increases, it makes the mass <strong>of</strong><strong>water</strong> injected increases. But, both volumes are the same. Thus, the <strong>temperature</strong>change <strong>of</strong> <strong>water</strong> injected must be decreases and led to decrease in the <strong>temperature</strong> <strong>of</strong>gases burned.4.6.1.3 Simulation with variation <strong>of</strong> engine speed. This simulation test wasrun at 2,000 rpm, equivalence ratio 0.8 while using the molar <strong>water</strong> <strong>injection</strong>-fuelratio such as 0.1 , 0.15 and 0.2 . These applications were used the different enginespeed (RPM) varied from 1000, 1500, 2000 and 2500, respectively and followed theschematic from Figure B-1 and assumption.