SUMMER 2023
Distributor's Link Magazine Summer 2023 / Vol 46 No 3
Distributor's Link Magazine Summer 2023 / Vol 46 No 3
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
130<br />
THE DISTRIBUTOR’S LINK<br />
ROB LaPOINTE FASTENER SCIENCE SPECTROSCOPY – THE ELEMENTAL CODE BEHIND THE CHEMISTRY OF METAL from page 90<br />
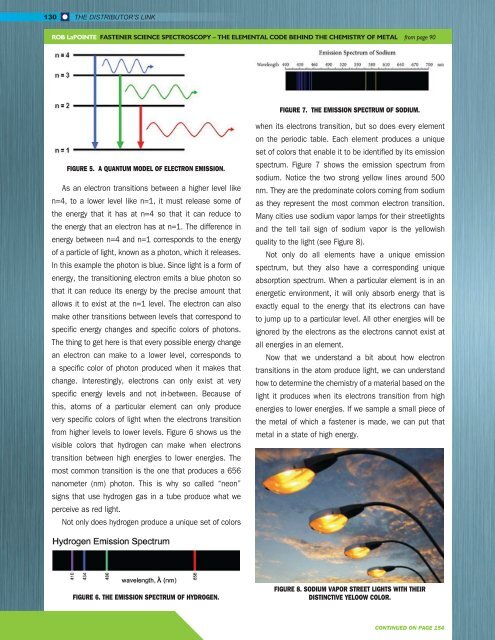
FIGURE 7. THE EMISSION SPECTRUM OF SODIUM.<br />
FIGURE 5. A QUANTUM MODEL OF ELECTRON EMISSION.<br />
As an electron transitions between a higher level like<br />
n=4, to a lower level like n=1, it must release some of<br />
the energy that it has at n=4 so that it can reduce to<br />
the energy that an electron has at n=1. The difference in<br />
energy between n=4 and n=1 corresponds to the energy<br />
of a particle of light, known as a photon, which it releases.<br />
In this example the photon is blue. Since light is a form of<br />
energy, the transitioning electron emits a blue photon so<br />
that it can reduce its energy by the precise amount that<br />
allows it to exist at the n=1 level. The electron can also<br />
make other transitions between levels that correspond to<br />
specific energy changes and specific colors of photons.<br />
The thing to get here is that every possible energy change<br />
an electron can make to a lower level, corresponds to<br />
a specific color of photon produced when it makes that<br />
change. Interestingly, electrons can only exist at very<br />
specific energy levels and not in-between. Because of<br />
this, atoms of a particular element can only produce<br />
very specific colors of light when the electrons transition<br />
from higher levels to lower levels. Figure 6 shows us the<br />
visible colors that hydrogen can make when electrons<br />
transition between high energies to lower energies. The<br />
most common transition is the one that produces a 656<br />
nanometer (nm) photon. This is why so called “neon”<br />
signs that use hydrogen gas in a tube produce what we<br />
perceive as red light.<br />
Not only does hydrogen produce a unique set of colors<br />
when its electrons transition, but so does every element<br />
on the periodic table. Each element produces a unique<br />
set of colors that enable it to be identified by its emission<br />
spectrum. Figure 7 shows the emission spectrum from<br />
sodium. Notice the two strong yellow lines around 500<br />
nm. They are the predominate colors coming from sodium<br />
as they represent the most common electron transition.<br />
Many cities use sodium vapor lamps for their streetlights<br />
and the tell tail sign of sodium vapor is the yellowish<br />
quality to the light (see Figure 8).<br />
Not only do all elements have a unique emission<br />
spectrum, but they also have a corresponding unique<br />
absorption spectrum. When a particular element is in an<br />
energetic environment, it will only absorb energy that is<br />
exactly equal to the energy that its electrons can have<br />
to jump up to a particular level. All other energies will be<br />
ignored by the electrons as the electrons cannot exist at<br />
all energies in an element.<br />
Now that we understand a bit about how electron<br />
transitions in the atom produce light, we can understand<br />
how to determine the chemistry of a material based on the<br />
light it produces when its electrons transition from high<br />
energies to lower energies. If we sample a small piece of<br />
the metal of which a fastener is made, we can put that<br />
metal in a state of high energy.<br />
FIGURE 6. THE EMISSION SPECTRUM OF HYDROGEN.<br />
FIGURE 8. SODIUM VAPOR STREET LIGHTS WITH THEIR<br />
DISTINCTIVE YELOOW COLOR.<br />
CONTINUED ON PAGE 154